Diseases of Swine (8th edition)/Chapter 17
Porcine parvovirus (PPV) causes reproductive failure of swine characterized by embryonic and fetal infection and death, usually in the absence of outward maternal clinical signs. The disease develops mainly when seronegative dams are exposed oronasally to the virus anytime during about the first half of gestation, and conceptuses are subsequently infected transplacentally before they become immunocompetent. There is no definitive evidence that infection of swine other than during gestation is of any clinical or economic significance. The virus is ubiquitous among swine throughout the world and is enzootic in most herds that have been tested. Diagnostic surveys have indicated that PPV is the major infectious cause of embryonic and fetal death (Cartwright and Huck 1967; Mengeling 1978b; Thacker and Leman 1978; Vannier and Tillon 1979; Mengeling et al. 1991).
ETIOLOGY
[edit]PPV is classified in the genus Parvovirus (Latin parvus = small) of the family Parvoviridae (Siegl 1976; Bachmann et al. 1979). All isolates of PPV that have been compared have been found antigenically similar if not identical (Cartwright et al. 1969; Johnson and Collings 1969; Morimoto et al. 1972a; Johnson et al. 1976; Ruckerbauer et al. 1978). PPV is also antigenically related to several other members of the genus (Cotmore et al. 1983; Mengeling et al. 1986, 1988). However, its identity can be established by relatively stringent serologic tests such as serum neutralization (SN) and hemagglutination inhibition (HI).
Biophysical and Biochemical Properties
[edit]The biophysical and biochemical properties of PPV have been extensively studied (Siegl 1976; Molitor et al. 1983; Berns 1984) and are summarized as follows. A mature virion has cubic symmetry, two or three capsid proteins, a diameter of approximately 20 nm, 32 capsomeres, no envelope or essential lipids, and a weight of 5.3 × 106 daltons. The viral genome is single-stranded deoxyribonucleic acid (DNA) with a molecular weight of 1.4 × 106 (i.e., about 26.5% of the weight of the complete virion). Buoyant densities (g/mL in cesium chloride) of complete infectious virions, incomplete "empty" virions, and extracted virion DNA are 1.38-1.395, 1.30-1.315, and 1.724 respectively. Viral infectivity, hemagglutinating activity, and antigenicity are remarkably resistant to heat, a wide range of hydrogen ion concentrations, and enzymes.
Replication
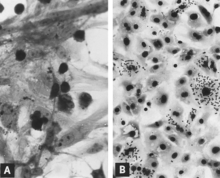
[edit]Replication of PPV in vitro is cytocidal and characterized by "rounding up," pyknosis, and lysis of cells (Fig. 17.1A). Many of the cell fragments often remain attached, eventually giving the affected culture a ragged appearance. Intranuclear inclusions develop (Cartwright et al. 1969) but they are often sparsely distributed (Rondhuis and Straver 1972). Infected cultures may hemadsorb slightly (Cartwright et al. 1969) (Fig. 17.1B). Cytopathic changes are extensive when cell culture-adapted virus is propagated under appropriate conditions. However, on initial isolation several serial passages of the virus (Cartwright et al. 1969) or, better, the infected culture may be necessary before the effects are recognized. The use of immunofluorescence (IF) microscopy greatly increases the likelihood of detecting minimally infected cultures (Lucas and Napthine 1971; Mengeling 1975).
Primary and secondary cultures of fetal or neonatal porcine kidney cells are most often used for propagation and titration of PPV, although other kinds of cultures are also susceptible (Pirtle 1974). Replication is enhanced by infection of mitotically active cultures (Mayr et al. 1968; Cartwright et al. 1969; Bachmann 1972; Hallauer et al. 1972). Many cells in such cultures are in the S phase (i.e., the DNA synthesis phase) of their cell cycle, wherein the DNA polymerases of cell origin needed for viral replication are available (Tennant 1971; Siegl and Gautschi 1973a, b).
If either fetal or adult bovine serum is incorporated in the nutrient medium of cell cultures used to propagate PPV, it should be pretested for viral inhibitors (Coackley and Smith 1972; Johnson 1973; Pini 1975). The same may apply to sera of several other species (Joo et al. 1976d). Because replication of PPV is affected by mitotic activity, the effect of the serum on the cells is also especially important. In addition, cultures should be pretested for PPV contamination (Lucas and Napthine 1971; Mengeling 1975). Cultures are sometimes unknowingly prepared from infected tissues of fetal (Mengeling 1975) and postnatal (Huygelen and Peetermans 1967; Bachmann 1969; Cartwright et al. 1969; Hafez and Liess 1979) pigs. Moreover, PPV can be accidentally introduced into cultures in several ways (Hallauer et al. 1971), including the use of contaminated trypsin (Croghan and Matchett 1973; Croghan et al. 1973). If contamination is detected before all cells are infected, the virus can be eliminated by repeatedly subculturing the cells in the presence of nutrient medium containing PPV antiserum (Mengeling 1978a).
Several investigators have used IF microscopy to follow the development of PPV in cell culture (Cartwright et al. 1969; Lucas and Napthine 1971; Mengeling 1972; Siegl et al. 1972; Bachmann and Danner 1976). In general, the sequence of events is as follows. Viral antigen is detected in the cytoplasm of cells soon after infection if the inoculum contains a high titer of virus and viral antigen. Most, if not all, of this early cytoplasmic fluorescence is the result of antigen phagocytized from the inoculum (Mengeling 1972; Mengeling and Cutlip 1975). By sequential examinations, such antigen can be demonstrated first on the external surface of the cytoplasmic membrane and later within the cytoplasm, often relatively concentrated in a juxtanuclear location. The first unequivocal evidence of viral replication is the appearance of nascent viral antigen in the nucleus (Fig. 17.2A). In at least some infected cells, nascent antigen next appears in the cytoplasm in sufficient quantity that both cytoplasm and nucleus are brightly fluorescent. Infected cells commonly seen in the lung of fetuses that develop a high titer of antibody for PPV probably represent this stage of replication (see Fig. 17.8C). Affected cells subsequently round up, become pyknotic, and disintegrate with release of virus and viral antigen (Fig. 17.2B). Other cells in the culture that are not at the appropriate stage to support viral replication continue to phagocytize and accumulate viral antigen in their cytoplasm (Fig. 17.2C). A second wave of viral replication can be induced if these cells are stimulated to enter the S phase of the cell cycle as, for example, by the addition of fresh culture medium.
Hemagglutination
[edit]PPV agglutinates human, monkey, guinea pig, cat, chicken, rat, and mouse erythrocytes. Erythrocytes of other kinds of animals that have been tested are relatively or completely insensitive, or the results have been equivocal (Darbyshire and Roberts 1968; Mayr et al. 1968; Cartwright et al. 1969; Hallauer et al. 1972; Mengeling 1972; Morimoto et al. 1972a). Several parameters of the hemagglutination (HA) test—such as the temperature of incubation (Mayr et al. 1968; Mengeling 1972), the species of erythrocyte used, and in the case of chicken erythrocytes the genetic composition (Cartwright et al. 1969; Pini 1975; Ruckerbauer et al. 1978) and age (Morimoto et al. 1972a) of the donor—may quantitatively affect results. The HA test is most commonly conducted at room temperature, at approximately neutral pH, and with guinea pig erythrocytes. Higher HA titers have been recorded when the diluent used in the test was veronal buffer rather than phosphate-buffered saline (Ruckerbauer et al. 1978). Elution of virus (the hemagglutinin is part of the virion) can be induced by suspending erythrocytes in alkaline buffer, pH 9 (Hallauer et al. 1972).
Infectivity Titrations
[edit]Infectivity titrations are conducted in a standard manner except that, because cytopathic changes at terminal dilutions are often vague, endpoints of infectivity are often determined either by examining cell cultures for intranuclear inclusions after appropriate staining or by examining cell culture medium for viral hemagglutinin (Cartwright et al. 1969). A titration procedure wherein infected cells are made evident by IF microscopy (Mengeling 1972) and a plaque assay (Kawamura et al. 1988) also have been described.

Serologic
[edit]Tests The HI test is frequently used for detection and quantitation of humoral antibody for PPV. Antibody sometimes can be detected as early as 5 days after swine are exposed to live virus, and it may persist for years (Johnson et al. 1976). Sera examined by the HI test are usually pretreated by heat inactivation (56˚C, 30 minutes) and by adsorption with erythrocytes (to remove naturally occurring hemagglutinins) and kaolin (to remove or reduce nonantibody inhibitors of HA) (Mengeling 1972; Morimoto et al. 1972a). Trypsin also has been used to remove nonantibody inhibitors of HA (Cartwright et al. 1969). Parameters of the HI test have been studied in detail (Kim 1974; Joo et al. 1976c).
The SN test is occasionally used for detection and quantitation of humoral antibody for PPV. Neutralization of infectivity is usually confirmed by the absence or reduction either of intranuclear inclusions or fluorescent cells in cultures or of viral hemagglutinin in the culture medium (Mengeling 1972; Johnson 1973; Joo et al. 1975). The SN test has been reported to be more sensitive than the HI test (Johnson and Collings 1971; Joo et al. 1975). A microtechnique for application of the SN test has been described (Joo et al. 1975).
Immunodiffusion (Joo et al. 1978), a modified direct complement-fixation test (Ruckerbauer et al. 1978), and enzyme-linked immunosorbent assay (Hohdatsu et al. 1988; Westenbrink et al. 1989) also have been used successfully to detect antibody for PPV.
EPIDEMIOLOGY
[edit]Porcine parvovirus is ubiquitous among swine throughout the world. In major swine-producing areas such as the midwestern United States, infection is enzootic in most herds, and with few exceptions sows are immune. In addition, a large proportion of gilts are naturally infected with PPV before they conceive, and as a result they develop an active immunity that probably persists throughout life. Collectively, the seroepidemiological data indicate that exposure to PPV is common. They also emphasize the high risk of infection and reproductive disease among gilts that have not developed immunity before conception. The most common routes of infection for postnatal and prenatal pigs are oronasal and transplacental respectively.
Pigs nursing immune dams absorb a high titer of antibody for PPV from colostrum. These titers decrease progressively with time by dilution as pigs grow as well as by biological degradation. They usually reach subdetectable levels in 3-6 months if sera are examined by the HI test (Etoh et al. 1979; Paul et al. 1982). Sometimes passively acquired antibody persists for a longer interval. Moreover, levels of antibody too low to be detected by the HI test may be detected by the SN test (Johnson et al. 1976). The primary significance of passively acquired antibody is that it interferes with the development of active immunity. High levels of such antibody can prevent infection, and lower levels can minimize dissemination from infected pigs (Suzuki and Fujisaki 1976; Paul et al. 1980). Consequently, some groups of gilts are not fully susceptible to infection and dissemination of virus until either shortly before conception or during early gestation.
Contaminated premises are probably major reservoirs of PPV. The virus is thermostable, is resistant to many common disinfectants (Brown 1981), and may remain infectious for months in secretions and excretions from acutely infected pigs. It was shown experimentally that although pigs transmitted PPV for only about 2 weeks after exposure, the pens in which they were initially kept remained infectious for at least 4 months (Mengeling and Paul 1986). The ubiquity of PPV also raises the possibility that some pigs are persistently infected and at least periodically shed virus. However, shedding beyond the interval of acute infection has not been demonstrated (Johnson et al. 1976). The possibility of immunotolerant carriers of PPV as a result of early in utero infection has been suggested (Johnson 1973). When gilts were infected with PPV before day 55 of gestation, their pigs were born infected but without antibody. Virus was isolated from kidneys, testicles, and seminal fluid of such pigs killed at various times after birth up to the time they were 8 months of age; at which time the experiment was terminated (Johnson and Collings 1971). Results of another study, wherein dams were infected early in gestation and their pigs were born infected but without antibody, also suggest an acquired immunotolerance (Cartwright et al. 1971). A possible example of an infected, immunotolerant, sexually active boar was reported (Johnson et al. 1976).
Boars may play a significant role in dissemination of PPV at a critical time. During acute infection the virus is shed by various routes, including semen, and the isolation of PPV from semen of naturally infected boars has been reported (Cartwright and Huck 1967; Cartwright et al. 1969; McAdaragh and Anderson 1975). Semen may be contaminated externally, as for example with viruscontaining feces, or within the male reproductive tract. The virus was isolated from a testicle of a boar 5 days after it was injected into the boarÕs prepuce (Lucas et al. 1974) and from testicles of boars killed 5 and 8 days after they were infected oronasally (Mengeling, unpublished data_1976). Virus was also isolated from scrotal lymph nodes of boars killed 5, 8, 15, 21, and 35 days after oronasal exposure. After day 8, isolation was accomplished by cocultivating lymph node fragments with fetal porcine kidney cells (Mengeling, unpublished data_ 1976). Irrespective of their immune status, boars can also function as a vehicle for mechanical dissemination of PPV among susceptible females.
CLINICAL SIGNS
[edit]Acute infection of postnatal pigs, including pregnant dams that subsequently develop reproductive failure, is usually subclinical (Johnson and Collings 1969; Cutlip and Mengeling 1975a; Fujisaki et al. 1975; Johnson et al. 1976; Joo et al. 1976a; Mengeling and Cutlip 1976). However, in young pigs and probably in older breeding stock as well, the virus replicates extensively and is found in many tissues and organs with a high mitotic index. Viral antigen is especially concentrated in lymphoid tissues (Cutlip and Mengeling 1975a; Fujisaki et al. 1975) (Fig. 17.3A, B). Many pigs, irrespective of age or sex, have a transient, usually mild, leukopenia sometime within 10 days after initial exposure to the virus (Johnson and Collings 1969, 1971; Joo et al. 1976a; Mengeling and Cutlip 1976). PPV and other structurally similar viruses have been identified in the feces of pigs with diarrhea (Dea et al. 1985; Yasuhara et al. 1989). However, there is no experimental evidence to suggest that PPV either replicates extensively in the intestinal crypt epithelium or causes enteric disease as do parvoviruses of several other species (Cutlip and Mengeling 1975a; Brown et al. 1980). PPV also has been isolated from pigs with lesions described as vesiclelike. The etiologic role of PPV in such lesions has not been clearly defined (Kresse et al. 1985).
The major and usually only clinical response to infection with PPV is maternal reproductive failure. Pathologic sequelae depend mainly on when exposure occurs during gestation. Dams may return to estrus, fail to farrow despite being anestrus, farrow few pigs per litter, or farrow a large proportion of mummified fetuses. All can reflect embryonic or fetal death or both. The only outward sign may be a decrease in maternal abdominal girth when fetuses die at midgestation or later and their associated fluids are resorbed. Other manifestations of maternal reproductive failure, namely, infertility, abortion, stillbirth, neonatal death, and reduced neonatal vitality, also have been ascribed to infection with PPV (Cartwright and Huck 1967; Johnson 1969; Morimoto et al. 1972b; Narita et al. 1975; Forman et al. 1977). These are normally only a minor component of the disease. The presence of mummified fetuses in a litter can prolong both gestation (Narita et al. 1975) and the farrowing interval (Mengeling et al. 1975). Either may result in stillbirth of apparently normal littermates, whether or not they are infected.

There is no evidence that either fertility or libido of boars is altered by infection with PPV (Biront and Bonte 1983; Thacker et al. 1987).
PATHOGENESIS
[edit]Dams are susceptible to PPV-induced reproductive failure if infected anytime during about the first half of gestation. This interval of maternal susceptibility is indicated by the collective results of several experimental studies (Joo et al. 1976a; Mengeling and Cutlip 1976; Mengeling 1979; Mengeling et al. 1980a), by in-depth epidemiological investigations (Donaldson-Wood et al. 1977; Gillick 1977), and by estimates of the time of death of fetuses collected during epidemiological surveys (Mengeling 1978b; Mengeling et al. 1991). Consequences of maternal infection during this interval are embryonic and fetal death followed by resorption and mummification respectively. Transplacental infection also follows maternal exposure after midgestation, but fetuses usually survive without obvious clinical effects in utero. The likely reason is that transplacental infection often requires 10–14 days (Mengeling et al. 1978, 1980a) or longer (Joo et al. 1976a), and by 70 days of gestation most fetuses are able to develop a protective immunologic response to the virus. In general, fetuses experimentally infected by transuterine inoculation of the virus have died when infected before day 70 of gestation, but they have survived and produced antibody when infected later in gestation (Redman et al. 1974; Bachmann et al. 1975; Cutlip and Mengeling 1975b; Mengeling and Cutlip 1975). A strain of PPV of slightly greater virulence also has been reported (Choi et al. 1987). The usual consequences of infection at different stages of gestation are summarized in Table 17.1.
When only part of a litter is infected transplacentally, as is often the case, one or more littermates are frequently infected by subsequent intrauterine spread of virus. The same would apply if initial infection were through contaminated semen. As a result, any combination or all of the sequelae indicated in Table 17.1 can develop in the same litter. Intrauterine dissemination is probably less common when early embryos are infected because they are quickly resorbed after death, effectively removing the intrauterine reservoir of virus (Mengeling et al. 1980a). In such cases there is no evidence at farrowing for the cause of fewer pigs per litter.
| Interval of Gestation(days)a | |||
|---|---|---|---|
| Infection of Dam | Infection of Conceptusb | Description of Conceptus | Consequences of Infection |
| ≤56 | 10–30 | Embryo | Death and resorption |
| 30–70 | Fetus | Death and mummification | |
| >56 | 70–term | Fetus | Immune response and usually survival in utero |
aIntervals are approximations.
bAssuming transplacental infections 10–14 days after maternal exposure.
The effect, if any, of PPV on the ovum before ovulation is unknown. The virus adheres tenaciously to the external surface of the zona pellucida of the fertilized porcine ovum (Wrathall and Mengeling 1979a, b), and although it apparently cannot penetrate this layer, speculation is that it could pose a threat to the embryo after hatching (Wrathall and Mengeling 1979a).
Despite strong circumstantial evidence (Cartwright et al. 1971), a direct causal role of PPV-contaminated semen in reproductive failure has not been established unequivocally (Lucas et al. 1974). The zona pellucida could protect the early embryo while local immunity is developing. Conversely, the virus may cause uterine changes incompatible with gestation (Wrathall and Mengeling 1979c). In any event, a female infected through semen provides a focus of infection for others.
With the possible exception of the uterine changes alluded to in the preceding paragraph, PPV-induced reproductive failure is caused by the direct effect of the virus on the conceptus. In the absence of an immune response, the virus replicates extensively throughout these tissues. By the time the conceptus dies, most of its cells contain large quantities of intracytoplasmic viral antigen that can be demonstrated by IF microscopy. The relative lack of nuclear fluorescence at the time of death, compared to earlier stages of the disease, indicates that when the conceptus is severely affected, mitotic activity and the associated conditions necessary for viral replication are suppressed more than phagocytic activity.
Death of the conceptus probably results from the collective damage by the virus to a variety of tissues and organs, including the placenta (Cutlip and Mengeling 1975b). However, in the absence of an immune response, changes in almost any vital organ are probably sufficient to eventually cause death. One of the most striking features of viral distribution is the extensive involvement of endothelium. This seems to preclude further development of the vascular network of the conceptus. Preparation for cellular mitosis (i.e., the S phase) results in concomitant viral replication and cell death. Damage to the fetal circulatory system is indicated by edema, hemorrhage, and the accumulation of large amounts of serosanguineous fluids in body cavities. Necrosis of the endothelium is microscopically evident (Lenghaus et al. 1978).
The mechanism of transplacental infection has been investigated by using IF microscopy to identify infected cells in maternal and fetal tissues at progressively longer intervals after maternal oronasal exposure (Mengeling et al. 1978). Examination of tissues contiguous with the maternal-fetal junction revealed viral antigen in endothelial and mesenchymal cells of the chorion, with increasing involvement of these tissues at progressively later stages of gestation. Viral antigen was never detected unequivocally in either uterine epithelium or trophectoderm. Consequently, there was no evidence for maternalfetal transfer of the virus by replicating through these tissues. However, this route cannot be excluded, since only a small part of the total area of contact was examined. Transfer of the virus within macrophages has been considered (Paul et al. 1979). Whatever the route, maternal viremia seems a likely prerequisite for transplacental infection (Joo et al. 1976a; Mengeling and Cutlip 1976).
LESIONS
[edit]Neither macroscopic nor microscopic lesions have been reported for nonpregnant pigs (Cutlip and Mengeling 1975a; Brown et al. 1980). It is conceivable that cellular infiltrations subsequently described for fetuses could be induced by infection during the perinatal interval.
Macroscopic lesions have not been reported in pregnant dams; however, microscopic lesions have been seen in tissues of gilts killed after their fetuses were infected by transuterine inoculation of virus. Gilts that were seronegative when their fetuses were infected at 70 days of gestation had focal accumulations of mononuclear cells adjacent to the endometrium and in deeper layers of the lamina propria when they were killed 12 and 21 days later. In addition, there were perivascular cuffs of plasma cells and lymphocytes in the brain, spinal cord, and choroid of the eye (Hogg et al. 1977). When fetuses were infected earlier in gestation (35, 50, and 60 days) and their dams were killed 7 and 11 days later, the lesions were similar. However, uterine lesions were more severe and also included extensive cuffing of myometrial and endometrial vessels with mononuclear cells (Lenghaus et al. 1978). Only focal accumulations of lymphocytes were seen in uteruses of gilts that were seropositive when their fetuses were infected (Cutlip and Mengeling 1975b).
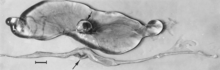
Macroscopic changes of embryos are death followed by resorption of fluids (Fig. 17.4) and then soft tissues (Fig. 17.5). Virus and viral antigen are widely distributed in tissues of infected embryos and their placentas (Mengeling et al. 1980a), and it is probable that microscopic lesions of necrosis and vascular damage, subsequently described for fetuses, also develop in advanced embryos.
There are numerous macroscopic changes in fetuses infected before they become immunocompetent (Fig. 17.6). These include a variable degree of stunting and sometimes an obvious loss of condition before other external changes are apparent; occasionally, an increased prominence of blood vessels over the surface of the fetus due to congestion and leakage of blood into contiguous tissues; congestion, edema, and hemorrhage with accumulation of serosanguineous fluids in body cavities; hemorrhagic discoloration becoming progressively darker after death; and dehydration (mummification). Many of these changes also apply to the placenta. Microscopic lesions consist primarily of extensive cellular necrosis in a wide variety of tissues and organs (Joo et al. 1977; Lenghaus et al. 1978) (Fig. 17.7A). Inflammation (Joo et al. 1977) and intranuclear inclusions (Lenghaus et al. 1978) also have been described.

In contrast, macroscopic changes have not been reported for fetuses infected after they become immunocompetent for PPV. Microscopic lesions are primarily endothelial hypertrophy (Hogg et al. 1977) and mononuclear cell infiltrations consistent with an immune response (Hogg et al. 1977; Joo et al. 1977). Meningoencephalitis characterized by perivascular cuffing with proliferating adventitial cells, histiocytes, and a few plasma cells was seen in the gray and white matter of the cerebrum and in the leptomeninges of PPV-infected stillborn pigs. These lesions were believed to be pathognomonic for PPV infection (Narita et al. 1975). Similar lesions have been observed in PPV-infected, live fetuses collected late in gestation (Hogg et al. 1977; Joo et al. 1977) (Fig. 17.7B).
Both general types of microscopic lesions (i.e., necrosis and mononuclear cell infiltration) may develop in fetuses infected near midgestation (Lenghaus et al. 1978) when the immune response is insufficient to provide protection.
DIAGNOSIS
[edit]PPV should be considered in a differential diagnosis of reproductive failure of swine whenever there is evidence of embryonic or fetal death or both. The pathologic sequelae of maternal infection during gestation have been described (see the section on clinical signs). If gilts but not sows are affected, maternal illness is not seen during gestation, there are few or no abortions or fetal developmental anomalies, and other evidence suggests an infectious disease, then a tentative diagnosis of PPV-induced reproductive failure can be made. The relative lack of maternal illness, abortions, and fetal developmental anomalies differentiates PPV from most other infectious causes of reproductive failure. However, a definitive diagnosis requires laboratory support.
Several mummified fetuses (<16 cm in length) or lungs from such fetuses, if sufficiently developed, should be submitted to the diagnostic laboratory. Larger mummified fetuses (i.e., more than about 70 days of gestational age) (Marrable and Ashdown 1967), stillborn pigs, and neonatal pigs are not recommended for submission unless they are the only samples available. If infected, their tissues will usually contain antibody that interferes with laboratory tests for either virus or viral antigen.

If females fail to farrow despite being anestrus and are sent to an abattoir, their uteruses should be collected and examined for affected fetuses. Sometimes only remnants of fetal tissues remain when fetuses die early in the middle third of gestation. Nevertheless, these are adequate samples if tested for viral antigen by IF microscopy (Mengeling and Cutlip 1975; Mengeling 1978b). The absence of affected fetuses or fetal remnants does not exclude PPV-induced reproductive failure. When all embryos of a litter die and are completely resorbed after the first few weeks of gestation, the dam may remain endocrinologically pregnant and not return to estrus until after the expected time of farrowing (Rodeffer et al. 1975).
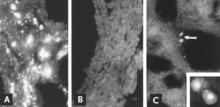
Identification of viral antigen by IF microscopy is a reliable and sensitive diagnostic procedure. Sections of fetal tissues are prepared with a cryostat microtome and are then reacted with standardized reagents (Mengeling et al. 1975; Mengeling 1978b). The test can be completed within a few hours. In the absence of a fetal antibody response, antigen is seen throughout fetal tissues (Fig. 17.8A, B); even when antibody is present, infected cells usually can be detected in fetal lung (Fig. 17.8C).
Detection of viral hemagglutinin also has been recommended as a diagnostic technique (Joo et al. 1976b; Joo and Johnson 1977a). Tissues are triturated in diluent and then sedimented by centrifugation. The supernatant fluid is tested for agglutinating activity for guinea pig erythrocytes. This test requires a minimum of laboratory equipment and is effective in the absence of antibody.

Virus isolation is less suitable as a routine diagnostic procedure than either of the aforementioned tests. Infectivity is slowly but progressively lost after fetal death (Mengeling and Cutlip 1975); as a result, isolation of virus from mummified fetuses that have died as a result of infection is sometimes unsuccessful (Mengeling 1978b). Moreover, the procedure is time-consuming, and contamination is a constant threat because of the stability of PPV in the laboratory (Cartwright et al. 1969) and because cell cultures are sometimes unknowingly prepared from infected tissues (Huygelen and Peetermans 1967; Bachmann 1969; Cartwright et al. 1969; Mengeling 1975; Hafez and Liess 1979). IF microscopy is often used to determine whether PPV has been isolated in cell culture (Cartwright 1970; Johnson 1973; Mengeling 1978b).
In general, serologic procedures are recommended for diagnosis only when tissues from mummified fetuses are not available for testing as previously described. Results with maternal sera are of value if antibody is not detected, thus excluding PPV as a cause, and if samples collected at intervals reveal seroconversion for PPV coincident with reproductive failure (Morimoto et al. 1972b; Mengeling et al. 1975; Rodeffer et al. 1975). Because PPV is ubiquitous, the presence of antibody in a single sample is otherwise meaningless. However, a determination of relative amounts of antibody present as immunoglobulin M and G can indicate the recency of infection (Kim 1974; Joo et al. 1978). Detection of antibody in sera of fetuses and stillborn pigs and in sera collected from neonatal pigs before they nurse is evidence of in utero infection, since maternal antibody does not cross the maternal-fetal junction (Johnson and Collings 1969, 1971; Cartwright et al. 1971; Mengeling 1972; Chaniago et al. 1978). When serum is not available, body fluids collected from fetuses or their viscera that have been kept in a plastic bag overnight at 4˚C have been used successfully to demonstrate antibody (Cropper et al. 1976; Joo et al. 1976b).
TREATMENT AND PREVENTION
[edit]There is no treatment for PPV-induced reproductive failure.
Gilts should be either naturally infected with PPV or vaccinated for PPV before they are bred. To promote natural infection, a common practice is to arrange contact between seronegative gilts and seropositive sows, with the expectation that one or more of the sows will be shedding virus. Moving gilts to a potentially contaminated area, either currently or recently inhabited by seropositive swine, also can be recommended. Once infection is started, the virus spreads rapidly among fully susceptible swine. Just how effective these procedures are in increasing the incidence of natural infection is unknown. For whatever reasons, infection is common, and probably well over one-half of all gilts in areas where PPV is enzootic are infected before they are bred for the first time (Mengeling 1972).
The use of vaccine is the only way to ensure that gilts develop active immunity before conception. Both inactivated (Suzuki and Fujisaki 1976; Ide et al. 1977; Joo and Johnson 1977b; Mengeling 1977; Fujisaki 1978; Fujisaki et al. 1978b; Mengeling et al. 1979, 1980b) and modified live-virus (MLV) vaccines (Paul and Mengeling 1980; Fujisaki and Murikami 1982) have been developed. An inactivated vaccine has been tested under field conditions (Fujisaki 1978; Fujisaki et al. 1978a), and both types of vaccines were effective when tested under controlled laboratory conditions (Mengeling et al. 1979, 1980b; Paul and Mengeling 1980).
Vaccines should be administered several weeks before conception to provide immunity throughout the susceptible period of gestation but after the disappearance of passively acquired colostral antibody, which could interfere with the development of active immunity (Paul and Mengeling 1986). These limits may define a very brief interval for effective vaccination of gilts that are bred before 7 months of age. Although inactivated vaccine provides maximum safety, there is experimental evidence that PPV can be sufficiently attenuated so that it is unlikely to cause reproductive failure even if inadvertently administered during gestation (Paul and Mengeling 1980). The apparent safety of MLV vaccine may be due to its reduced ability to replicate in tissues of the intact host and cause the level of viremia needed for transplacental infection (Paul and Mengeling 1984). Moreover, it has been shown by transuterine inoculation of both virulent and attenuated virus that a much larger dose of attenuated virus is required to establish infection of fetuses (Mengeling et al. 1984). Duration of immunity following vaccination is unknown; however, in one study antibody titers were maintained for at least 4 months after administration of an inactivated vaccine (Joo and Johnson 1977b). Low levels of antibody found to be protective allow speculation that, once the immune system has been primed with PPV, subsequent exposure to virulent virus during gestation is unlikely to result in transplacental infection even if antibody from vaccination is no longer detected (Mengeling et al. 1979).
Vaccination is recommended also for seronegative sows and boars. Seronegative sows are usually found only in PPV-free herds; in such cases, inactivated vaccine is indicated. Experience has shown that few herds can be expected to remain free of PPV even if access is carefully controlled. Introduction of PPV into a totally susceptible herd can be disastrous (Donaldson-Wood et al. 1977). Vaccination of boars should reduce their involvement in dissemination of the virus.
Vaccines are used extensively in the United States and in several other countries where PPV has been recognized as an economically important cause of reproductive failure. All federally licensed vaccines marketed in the United States are inactivated.
REFERENCES
[edit]Bachmann, P. A. 1969. Vorkommen und Verbreitung von Picodna (Parvo)-Virus beim Schwein. Zentralbl Veterinärmed (B) 16:341-345.
___. 1972. Porcine parvovirus infection in vitro: A study model for the replication of parvoviruses. I. Replication at different temperatures. Proc Soc Exp Biol Med 140:1369-1374.
Bachmann, P. A., and Danner, K. 1976. Porcine parvovirus infection in vitro: A study model for the replication of parvoviruses. II. Kinetics of virus and antigen production. Zentralbl Veterinärmed (B) 23:355-363.
Bachmann, P. A.; Sheffy, B. E.; and Vaughan, J. T. 1975. Experimental in utero infection of fetal pigs with a porcine parvovirus. Infect Immun 12:455-460.
Bachmann, P. A.; Hoggan, M. D.; Kurstak, E.; Melnick, J. L.; Pereira, H. G.; Tattersall, P.; and Vago, C. 1979. Parvoviridae: Second report. Intervirology 11:248-254.
Berns, K. I. 1984. The Parvoviruses. New York: Plenum.
Biront, P., and Bonte, P. 1983. Porcine parvovirus (P.P.V.) infection in boars. I. Possibility of a genital localization in the boar after oronasal infection. Zentralbl Veterinärmed 30:541-545.
Brown, T. T., Jr. 1981. Laboratory evaluation of selected disinfectants as virucidal agents against porcine parvovirus, pseudorabies virus, and transmissible gastroenteritis virus. Am J Vet Res 42:1033-1036.
Brown, T. T., Jr.; Paul, P. S.; and Mengeling, W. L. 1980. Response of conventionally raised weanling pigs to experimental infection with a virulent strain of porcine parvovirus. Am J Vet Res 41:1221-1224.
Cartwright, S. F. 1970. Tests available for the detection of some virus infections of pigs and their interpretation. Vet Annu 11:77-82.
Cartwright, S. F., and Huck, R. A. 1967. Viruses isolated in association with herd infertility, abortions and stillbirths in pigs. Vet Rec 81:196-197.
Cartwright, S. F.; Lucas, M.; and Huck, R. A. 1969. A small haemagglutinating porcine DNA virus. I. Isolation and properties. J Comp Pathol 79:371-377.
___. 1971. A small haemagglutinating porcine DNA virus. II. Biological and serological studies. J Comp Pathol 81:145-155.
Chaniago, T. D.; Watson, D. L.; Owen, R. A.; and Johnson, R. H. 1978. Immunoglobulins in blood serum of foetal pigs. Aust Vet J 54:30-33.
Choi, C. S.; Molitor, T. W.; Joo, H. S.; and Gunther, R. 1987. Pathogenicity of a skin isolate of porcine parvovirus in swine fetuses. Vet Microbiol 15:19-29.
Coackley, W., and Smith, V. W. 1972. Porcine parvoviruses in Western Australia. Aust Vet J 48:536.
Cotmore, S. F.; Sturzenbecker, L. J.; and Tattersall, P. 1983. The autonomous parvovirus MVM encodes two nonstructural proteins in addition to its capsid polypeptides. Virology 129:333-343.
Croghan, D. L., and Matchett, A. 1973. b-propiolactone sterilization of commercial trypsin. Appl Microbiol 26:832.
Croghan, D. L.; Matchett, A.; and Koski, T. A. 1973. Isolation of porcine parvovirus from commercial trypsin. Appl Microbiol 26:431-433.
Cropper, M.; Dunne, H. W.; Leman, A. D.; Starkey, A. L.; and Hoefling, D. C. 1976. Prevalence of antibodies to porcine enteroviruses and porcine parvovirus in body fluids of fetal pigs from small vs. large litters. J Am Vet Med Assoc 168:233-235.
Cutlip, R. C., and Mengeling, W. L. 1975a. Experimentally induced infection of neonatal swine with porcine parvovirus. Am J Vet Res 36:1179-1182.
___. 1975b. Pathogenesis of in utero infection of eightand ten-week-old porcine fetuses with porcine parvovirus. Am J Vet Res 36:1751-1754.
Darbyshire, J. H., and Roberts, D. H. 1968. Some respiratory virus and mycoplasma infections of animals. J Clin Pathol 21(Suppl 2):61-92.
Dea, S.; Elazhary, M. A. S. Y.; Martineau, G. P.; and Vaillancourt, J. 1985. Parvovirus-like particles associated with diarrhea in unweaned piglets. Can J Comp Med 49:343-345.
Donaldson-Wood, C. R.; Joo, H. S.; and Johnson, R. H. 1977. The effect on reproductive performance of porcine parvovirus infection in a susceptible pig herd. Vet Rec 100:237-239.
Etoh, M.; Morishita, E.; and Watanabe, Y. 1979. Transitional antibodies and spontaneous infection in porcine parvovirus infection. Jpn J Swine Husb Res 16:237-239.
Forman, A. J.; Lenghaus, C.; Hogg, G. G.; and Hale, C. J. 1977. Association of a parvovirus with an outbreak of foetal death and mummification in pigs. Aust Vet J 53:326-329.
Fujisaki, Y. 1978. Incidence and control of stillbirth caused by porcine parvovirus in Japan. Proc Int Congr Pig Vet Soc 5:KA 14.
Fujisaki, Y., and Murikami, Y. 1982. Immunity to infection with porcine parvovirus in pigs inoculated with attenuated HT-strain. Natl Inst Anim Health (Tokyo) 22:36-37.
Fujisaki, Y.; Morimoto, T.; Sugimori, T.; and Suziki, H. 1975. Experimental infection of pigs with porcine parvovirus. Natl Inst Anim Health Q (Tokyo) 22:205-206.
Fujisaki, Y.; Ichihara, T.; Sasaki, N.; Shimizu, F.; Murakami, Y.; Sugimori, T.; and Sasahara, J. 1978a. Field trials on inactivated porcine parvovirus vaccine for prevention of viral stillbirth among swine. Natl Inst Anim Health Q (Tokyo) 18:184-185.
Fujisaki, Y.; Watanabe, Y.; Kodama, K.; Hamada, H.; Murakami, Y.; Sugimori, T.; and Sasahara, J. 1978b. Protection of swine with inactivated porcine parvovirus vaccine from fetal infection. Natl Inst Anim Health Q (Tokyo) 18:185.
Gillick, J. C. 1977. An outbreak of swine foetal mummification associated with porcine parvovirus. Aust Vet J 53:105-106.
Hafez, S. M., and Liess, B. 1979. Isolation of parvovirus from kidney cell cultures of gnotobiotic piglets. Zentralbl Veterinärmed (B) 26:820-827.
Hallauer, C.; Kronauer, G.; and Siegl, G. 1971. Parvovirus as contaminants of permanent human cell lines. I. Virus isolations from 1960-1970. Arch Gesamte Virusforsch 35:80-90.
Hallauer, C.; Siegl, G.; and Kronauer, G. 1972. Parvoviruses as contaminants of permanent human cell lines. III. Biological properties of the isolated viruses. Arch Gesamte Virusforsch 38:369-382.
Hogg, G. G.; Lenghaus, C.; and Forman, A. J. 1977. Experimental porcine parvovirus infection of foetal pigs resulting in abortion, histological lesions and antibody formation. J Comp Pathol 87:539-549.
Hohdatsu, T.; Baba, K.; Ide, S.; Tsuchimoto, M.; Nagano, H.; Yamagami, T.; Yamagishi, H.; Fujisaki, Y.; and Matumoto, M. 1988. Detection of antibodies against porcine parvovirus in swine sera by enzyme-linked immunosorbent assay. Vet Microbiol 17:11-19.
Huygelen, C., and Peetermans, J. 1967. Isolation of a hemagglutinating picornavirus from a primary swine kidney cell culture. Arch Gesamte Virusforsch 20:260-262.
Ide, S.; Yamagishi, K.; Yoshimura, M.; Maniwa, E.; Yasuda, H.; and Igarashi, J. 1977. Reaction of pigs to injection with a bivalent vaccine of Japanese B encephalitis virus and porcine parvovirus. J Jpn Vet Med Assoc 30:322-325.
Johnson, R. H. 1969. A search for Parvoviridae (Picornaviridae). Vet Rec 84:19-20.
___. 1973. Isolation of swine parvovirus in Queensland. Aust Vet J 49:257-259.
Johnson, R. H., and Collings, D. F. 1969. Experimental infection of piglets and pregnant gilts with a parvovirus. Vet Rec 85:446-447.
___. 1971. Transplacental infection of piglets with a porcine parvovirus. Res Vet Sci 12:570-572.
Johnson, R. H.; Donaldson-Wood, C. R.; Joo, H. S.; and Allender, U. 1976. Observations on the epidemiology of porcine parvovirus. Aust Vet J 52:80-84.
Joo, H. S., and Johnson, R. H. 1977a. Observations on rapid diagnosis of porcine parvovirus in mummified foetuses. Aust Vet J 53:106-107.
___. 1977b. Serological responses in pigs vaccinated with inactivated porcine parvovirus. Aust Vet J 53:550-552.
Joo, H. S.; Donaldson-Wood, C. R.; and Johnson, R. H. 1975. A microneutralization test for the assay of porcine parvovirus antibody. Arch Virol 47:337-341.
___. 1976a. Observations on the pathogenesis of porcine parvovirus infection. Arch Virol 51:123-129.
___. 1976b. Rapid diagnostic techniques for detection of porcine parvovirus infection in mummified foetuses. Aust Vet J 52:51.
___. 1976c. A standardised haemagglutination inhibition test for porcine parvovirus antibody. Aust Vet J 52:422-424.
Joo, H. S.; Donaldson-Wood, C. R.; Johnson, R. H.; and Watson, D. L. 1976d. Antibody to porcine, feline and rat parvoviruses in various animal species. Res Vet Sci 21:112-113.
Joo, H. S.; Donaldson-Wood, C. R.; Johnson, R. H.; and Campbell, R. S. F. 1977. Pathogenesis of porcine parvovirus infection: Pathology and immunofluorescence in the foetus. J Comp Pathol 87:383-391.
Joo, H. S.; Johnson, R. H.; and Watson, D. L. 1978. Serological procedures to determine time of infection of pigs with porcine parvovirus. Aust Vet J 54:125-127.
Kawamura, H.; Fujita, T.; and Imada, T. 1988. Plaque formation and replication of porcine parvovirus in embryonic swine kidney cell line, ESK cells. Jpn J Vet Sci 50:803-808.
Kim, Y. H. 1974. Studies on hemagglutination and hemagglutination- inhibition reaction of porcine parvovirus. Bull AZABU Vet Coll 27:61-65.
Kresse, J. I.; Taylor, W. D.; Stewart, W. C.; and Eernisse, K. A. 1985. Parvovirus infection in pigs with necrotic and vesicle- like lesions. Vet Microbiol 10:525-531.
Lenghaus, C.; Forman, A. J.; and Hale, C. J. 1978. Experimental infection of 35, 50 and 60 day old pig foetuses with porcine parvovirus. Aust Vet J 54:418-422.
Lucas, M. H., and Napthine, P. 1971. Fluorescent antibody technique in the study of three porcine viruses: Transmissible gastroenteritis virus, vomiting and wasting disease virus, and the parvovirus 59e/63. J Comp Pathol 81:111-117.
Lucas, M. H.; Cartwright, S. F.; and Wrathall, A. E. 1974. Genital infection of pigs with porcine parvovirus. J Comp Pathol 84:347-350.
Marrable, A. W., and Ashdown, R. R. 1967. Quantitative observations on pig embryos of known ages. J Agric Sci 69:443-447.
Mayr, A.; Bachmann, P. A.; Siegl, G.; Mahnel, H.; and Sheffy, B. E. 1968. Characterization of a small porcine DNA virus. Arch Gesamte Virusforsch 25:38-51.
McAdaragh, J. P., and Anderson, G. A. 1975. Transmission of viruses through boar semen. In Proc 18th Annu Meet Am Assoc Vet Lab Diagn, pp. 69-76.
Mengeling, W. L. 1972. Porcine parvovirus: Properties and prevalence of a strain isolated in the United States. Am J Vet Res 33:2239-2248.
___. 1975. Porcine parvovirus: Frequency of naturally occurring transplacental infection and viral contamination of fetal porcine kidney cell cultures. Am J Vet Res 36:41-44.
___. 1977. Diagnosing porcine parvovirus-induced reproductive failure. In Proc 20th Annu Meet Am Assoc Vet Lab Diagn, pp. 237-244.
___. 1978a. Elimination of porcine parvovirus from infected cell cultures by inclusion of homologous antiserum in the nutrient medium. Am J Vet Res 39:323-324.
___. 1978b. Prevalence of porcine parvovirus-induced reproductive failure: An abattoir study. J Am Vet Med Assoc 172:1291-1294.
___. 1979. Prenatal infection following maternal exposure to porcine parvovirus on either the seventh or fourteenth day of gestation. Can J Comp Med 43:106-109.
Mengeling, W. L., and Cutlip, R. C. 1975. Pathogenesis of in utero infection: Experimental infection of 5-week-old porcine fetuses with porcine parvovirus. Am J Vet Res 36:1173-1177.
___. 1976. Reproductive disease experimentally induced by exposing pregnant gilts to porcine parvovirus. Am J Vet Res 37:1393-1400.
Mengeling, W. L., and Paul, P. S. 1986. The relative importance of swine and contaminated premises as reservoirs of porcine parvovirus. J Am Vet Med Assoc 188:1293-1295.
Mengeling, W. L.; Cutlip, R. C.; Wilson, R. A.; Parks, J. B.; and Marshall, R. F. 1975. Fetal mummification associated with porcine parvovirus infection. J Am Vet Med Assoc 166:993-995.
Mengeling, W. L.; Cutlip, R. C.; and Barnett, D. 1978. Porcine parvovirus: Pathogenesis, prevalence, and prophylaxis. Proc Int Congr Pig Vet Soc 5:KA 15.
Mengeling, W. L.; Brown, T. T., Jr.; Paul, P. S.; and Guntekunst, D. E. 1979. Efficacy of an inactivated virus vaccine for prevention of porcine parvovirus-induced reproductive failure. Am J Vet Res 40:204-207.
Mengeling, W. L.; Paul, P. S.; and Brown, T. T., Jr. 1980a. Transplacental infection and embryonic death following maternal exposure to porcine parvovirus near the time of conception. Arch Virol 65:55-62.
Mengeling, W. L.; Paul, P. S.; Gutekunst, D. E.; Pirtle, E. C.; and Brown, T. T., Jr. 1980b. Vaccination for reproductive failure caused by porcine parvovirus. Proc Int Congr Pig Vet Soc 6:61.
Mengeling, W. L.; Pejsak, Z.; and Paul, P. S. 1984. Biological assay of attenuated strain NADL-2 and virulent strain NADL-8 of porcine parvovirus. Am J Vet Res 45:2403-2407.
Mengeling, W. L.; Paul, P. S.; Bunn, T. O.; and Ridpath, J. F. 1986. Antigenic relationships among autonomous parvoviruses. J Gen Virol 67:2839-2844.
Mengeling, W. L.; Ridpath, J. F.; and Vorwald, A. C. 1988. Size and antigenic comparisons among the structural proteins of selected autonomous parvoviruses. J Gen Virol 69:825-837.
Mengeling, W. L.; Lager, K. M.; Zimmerman, J. K.; Samarikermani, N.; and Beran, G. W. 1991. A current assessment of the relative role of porcine parvovirus as a cause of fetal porcine death. J Vet Diagn Invest 3:33-35.
Molitor, T. W.; Joo, H. S.; and Collect, M. S. 1983. Porcine parvovirus: Virus purification and structural and antigenic properties of virion polypeptides. J Virol 45:842-854.
Morimoto, T.; Fujisaki, Y.; Ito, Y.; and Tanaka, Y. 1972a. Biological and physiochemical properties of porcine parvovirus recovered from stillborn piglets. Natl Inst Anim Health Q (Tokyo) 12:137-144.
Morimoto, T.; Kurogi, H.; Miura, Y.; Sugimori, T.; and Fujisaki, Y. 1972b. Isolation of Japanese encephalitis virus and a hemagglutinating DNA virus from the brain of stillborn piglets. Natl Inst Anim Health Q (Tokyo) 12:127-136.
Narita, M.; Inui, S.; Kawakami, Y.; Kitamura, K.; and Maeda, A. 1975. Histopathological changes of the brain in swine fetuses naturally infected with porcine parvovirus. Natl Inst Anim Health Q (Tokyo) 15:24-28.
Paul, P. S., and Mengeling, W. L. 1980. Evaluation of a modified live virus vaccine for the prevention of porcine parvovirus- induced reproductive disease in pigs. Am J Vet Res 41:2007-2011.
___. 1984. Oronasal and intramuscular vaccination of swine with a modified live porcine parvovirus vaccine: Multiplication and transmission of vaccine virus. Am J Vet Res 45:2481-2485.
___. 1986. Vaccination of swine with inactivated porcine parvovirus vaccine in the presence of passive immunity. J Am Vet Med Assoc 188:410-413.
Paul, P. S.; Mengeling, W. L.; and Brown, T. T., Jr. 1979. Replication of porcine parvovirus in peripheral blood lymphocytes, monocytes, and peritoneal macrophages. Infect Immun 25:1003-1007.
___. 1980. Effect of vaccinal and passive immunity on experimental infection of pigs with porcine parvovirus. Am J Vet Res 41:1368-1371.
Paul, P. S.; Mengeling, W. L.; and Pirtle, E. C. 1982. Duration and biological half-life of passively acquired colostral antibodies to porcine parvovirus. Am J Vet Res 43:1376-1379.
Pini, A. 1975. Porcine parvovirus in pig herds in southern Africa. J S Afr Vet Assoc 46:241-244.
Pirtle, E. C. 1974. Titration of two porcine respiratory viruses in mammalian cell cultures by direct fluorescent antibody staining. Am J Vet Res 35:249-250.
Redman, D. R.; Bohl, E. H.; and Ferguson, L. C. 1974. Porcine parvovirus: Natural and experimental infections of the porcine fetus and prevalence in mature swine. Infect Immun 10:718-723.
Rodeffer, H. E.; Leman, A. D.; Dunne, H. W.; Cropper, M.; and Sprecher, D. J. 1975. Reproductive failure in swine associated with maternal seroconversion for porcine parvovirus. J Am Vet Med Assoc 166:991-995.
Rondhuis, P. R., and Straver, P. J. 1972. Enige kenmerken van een klien, hemagglutinerend DNA-virus, geisoleer uit een verworpen varkensfoetus. Tijdschr Diergeneeskd 97:1257-1267.
Ruckerbauer, G. M.; Dulac, G. C.; and Boulanger, P. 1978. Demonstration of parvovirus in Canadian swine and antigenic relationships with isolates from other countries. Can J Comp Med 42:278-285.
Siegl, G. 1976. The Parvoviruses, 1st ed. Vienna, Austria: Springer-Verlag.
Siegl, G., and Gautschi, M. 1973a. The multiplication of parvovirus Lu III in a synchronized culture system. I. Optimum conditions for virus replication. Arch Gesamte Virusforsch 40:105-118.
___. 1973b. The multiplication of parvovirus Lu III in a synchronized culture system. II. Biochemical characteristics of virus replication. Arch Gesamte Virusforsch 40:119-127.
Siegl, G.; Hallauer, C.; and Novak, A. 1972. Parvoviruses as contaminants of permanent human cell lines. IV. Multiplication of KBSH-virus in KB-cells. Arch Gesamte Virusforsch 36:351.
Suzuki, H., and Fujisaki, Y. 1976. Immunizing effects of inactivated porcine parvovirus vaccine on piglets. Natl Inst Anim Health Q (Tokyo) 16:81.
Tennant, R. W. 1971. Inhibition of mitosis and macromolecular synthesis in rat embryo cells by Kilhan rat virus. J Virol 8:402-408.
Thacker, B., and Leman, A. D. 1978. Evaluation of gravid uteri at slaughter for porcine parvovirus infection. Proc Int Congr Pig Vet Soc 5:M-49.
Thacker, B. J.; Joo, H. S.; Winkelman, N. L.; Leman, A. D.; and Barnes, D. M. 1987. Clinical, virologic, and histopathologic observations of induced porcine parvovirus infection in boars. Am J Vet Res 48:763-767.
Vannier, P., and Tillon, J. P. 1979. Diagnostic de certitude de l'infection a parvovirus dans les trouble de la reproduction de l'espèce porcine. Rec Med Vet 155:151-158.
Westenbrink, F.; Veldius, M. A.; and Brinkhof, J. M. A. 1989. An enzyme-linked assay for detection of antibodies to porcine parvovirus. J Virol Methods 23:169-178.
Wrathall, A. E., and Mengeling, W. L. 1979a. Effect of porcine parvovirus on development of fertilized pig eggs in vitro. Br Vet J 135:249-254.
___. 1979b. Effect of transferring parvovirus-infected fertilized pig eggs into seronegative gilts. B Vet J 135:255-261.
___. 1979c. Effect of inseminating seropositive gilts with semen containing porcine parvovirus. Br Vet 135:420-425.
Yasuhara, H.; Matsui, O.; Hirahara, T.; Ohgtani, T.; Tanaka, M. L.; Kodama, K.; Nakai, M; and Sasaki, N. 1989. Characterization of parvovirus isolated from diarrheic feces of a pig. Jpn J Vet Sci 51:337-344.
