1911 Encyclopædia Britannica/Rubber
RUBBER, Indiarubber or Caoutchouc (a word probably derived from Cahucha or Caucho the names in Ecuador and Peru respectively for rubber or the tree producing it), the chief constituent of the coagulated milky juice or latex furnished by a number of different trees, shrubs and vines. The latex of the best rubber plants furnishes from 20 to 50% of rubber. The latex is not to be confused with the sap of trees, on the circulation of which their nutrition depends. Though frequently occurring, it is not a universal feature of plant life, and does not appear to be necessary or even directly connected with the nutritive system of plants. Its exact function is not fully understood. Latex, though chiefly secreted in vessels or small sacs which reside in the cortical tissue between the outer bark and the wood is also found in the leaves and sometimes in the roots or bulbs. The trees and plants whose latices furnish caoutchouc in considerable quantity chiefly belong to the natural orders Euphorbiaceae, Urticaceae, Apocynaceae, Asclepiadaceae. The latex is usually obtained from the bark or stem by ma-king an incision reaching almost to the wood when the milky fluid flows more or less readily from the laticiferous vessels. It is, like milk, an emulsion, and when examined with the microscope is seen to consist"of numerous globules suspended in a watery fluid. On standing, some latices separate, more or less readily, into an upper layer resembling cream and consisting of the globules, and a lower watery layer. This separation can be rapidly effected with some latices by the use of a centrifugal machine, but this method has not yet been applied to any extent commercially. The globules which furnish the cream gradually pass on standing into solid caoutchouc, a process which is facilitated by rapid stirring, or by the addition of an acid or other chemical agent. If the latex is warmed or an acid, an alkali or astringent 'plant juice is added to it, “coagulation” usually takes place more or less readily, the caoutchouc separating in solid flakes or curds. The efficacy of heat or of an acid, an alkali or other agent in promoting coagulation depends on the character of the latex, and varies with that obtained from different plants. The watery fluid in which the globules are suspended holds certain proteids, carbohydrates and a small proportion of salts in solution. The latex exhibits a neutral, acid or alkaline reaction depending upon the plant from which it has been obtained.
When exposed to air the latex gradually undergoes putrefactive changes accompanied by coagulation of the caoutchouc. The addition of a small quantity of ammonia or of formalin to some latices usually has the effect of preserving them for a considerable time. The nature of the coagulation is not yet completely understood. It has been compared with that of milk and of blood, which depend essentially on the coagulation or separation in curds of a proteid or albuminous substance, such as takes place when white of egg is warmed. There is, however, reason to believe that the coagulation of latex into rubber is not mainly of this character. The globules in the latex are liquid, and the phenomenon of coagulation would seem to consist in the passage of this liquid into solid caoutchouc through the kind of change known as polymerization or condensation, in which a liquid passes into solid without alteration of composition or by condensation with the elimination of the elements of water. The effect of chemical agents in producing coagulation are in consonance with what is known of other instances of polymeric or condensation changes, whilst the fact that the collection of globules separated by creaming after thorough washing, and therefore removal of all proteid, is susceptible of solidification into caoutchouc by a merely mechanical act such as churning, strongly supports the view that the character of the change is distinct from that of any alteration which may occur in the proteid constituents of the latex.
The existence of caoutchouc or rubber was first observed soon after the discovery of America. It was noticed that certain Indian tribes of South America played with a ball composed of a resilient and elastic substance, which afterwards was found to possess the power of removing lead pencil marks from paper and came into commerce as “Indian Rubber.” It was not until the middle of the 18th century that the trees which yielded caoutchouc were identified, chiefly by French observers. La Condamine ascertained the nature of the tree, now known as Hevea brasiliensis, from which the Para rubber of S. America was obtained, whilst a little later Fresnau and Aublet described the Euphorbiaceous trees which furnished the rubber of Guiana.
The methods adopted by the natives in S. America and in Mexico for incising the trees and obtaining the rubber are exceedingly primitive, but survive with little modification at the present day.
Statistics of Rubber Production.—Until recently rubber was obtained almost exclusively from the tropical forests of S. and Central America, E. and W. Africa and Asia, being the produce of naturally occurring trees and vines. The increase in the demand, for which the employment of rubber tires is largely responsible, has given an increased stimulus to the production of “wild” rubber, with the result that trees and vines have been recklessly cut and destroyed, and in some instances vast regions, as in the S. Sudan, have been nearly entirely denuded of rubber vines. This has led to restrictive measures, the vines being tapped under definite regulations as to the manner and time of tapping, and also to requirements as to replanting vines to take the place of those which have been injured or destroyed, certain areas being periodically closed. Such measures, which are now in operation in the French Sudan, the Congo and in German W. and E. Africa, can, however, only be enforced by special administrative machinery and at considerable expense, and this legislative action can only be regarded as temporary and preliminary to the establishment of plantations of rubber trees, which are not only easier to control, but the trees are less liable to injury from careless tapping. In Africa it seems probable that the production of rubber from vines is likely to be entirely superseded in process of time, and replaced by the plantations of trees which are already being established in those districts in which careful experiment has determined the kind of rubber tree best adapted to the locality. The forests of tropical America have suffered similarly, trees having been injured or destroyed and in some cases cut down in order to secure the immediate increase of supply which was called for by a considerable rise in value. The result has been that in the forests of Brazil and Mexico the conservation of rubber trees has received greater attention, whilst new and extensive areas are planted in S. and Central America. The wild rubber of S. and Central America is still the principal source of the rubber supply of the world, and is likely to continue to be so for many years to come. Although the cost of transport from the remote forest regions of some districts is a serious consideration, this is not likely to be operative in reducing production until there has been a considerable and permanent fall in price, by which time new areas in those countries in which planting is now taking place will probably have come into bearing.
The enormous increase in the commercial demand for rubber and the probability of the continuance of this increase in view of the great variety of purposes to which the material can be applied, has led to great activity in rubber planting in other parts of the world, especially in Ceylon and the Malay Peninsula and Archipelago, where the Para rubber trees (Hevea brasiliensis) has been successfully introduced, and numerous plantations, many of which have not been in existence for more than ten or fifteen years, are now contributing to the world’s supply. This rubber is known as “Plantation” rubber in contradistinction to the “wild” rubber.
“Plantation” Para rubber from Ceylon and the Malay States has brought prices equal to and often exceeding those of fine Para rubber from Brazil. This is largely due to the improved methods of preparing the rubber practised by the planters of Ceylon and Malaya, which lead to the exclusion of the impurities usually found in “wild” rubber. Para rubber from Brazil generally contains about 15% of water, whilst “plantation” Para is usually nearly dry and contains 1% of water or less. It would appear, however, that the finest “wild” Para rubber as a rule possesses greater tensile strength than the “plantation” rubber. This has been ascribed by some to the presence in “wild” rubber of certain impurities derived either from the latex or introduced during the preparation of the rubber which are thought to enhance the physical properties of the caoutchouc. It is more probable, however, that the superiority of the “wild” Para is principally due to the greater age of the forest trees from which the rubber is obtained, many of which are from thirty to fifty years old. It is well known that the Hevea tree usually furnishes very inferior rubber if tapped before it is six or seven years old, and there is evidence to show that the quality of the rubber improves with the age of the tree. The oldest of the plantation trees of Ceylon and Malaya are not much more than twelve years old, whilst it is to be feared that immature trees are often tapped and their latex mixed with that of older trees before coagulation, thus forming inferior rubber. It is therefore to be expected that as time goes on the quality of “plantation” rubber will improve, and there would seem to be no reason why it should not eventually be fully equal to that of the “wild” rubber.
In 1909 the total production of rubber is stated to have been about 70,000 tons, of which more than one-half came from tropical America, about one-third from Africa, whilst the remainder was chiefly of Asiatic origin, including “plantation” rubber from Ceylon and Malaya, which amounted to about 3000 tons.
Chiefly owing to the supplies of “wild” rubber which are still available, comparatively little has been done until recently in establishing plantations either in Africa or in tropical America, but in Asia, including Ceylon, India and Malaya, in which there are relatively few important naturally-occurring rubber plants, there has been for some years great activity in forming plantations of rubber trees introduced mainly from tropical America, and there are now many millions sterling of British capital invested in companies established to form rubber plantations chiefly in Ceylon and Malaya. Each year should therefore show an increase in the production of plantation rubber. No trustworthy estimate of the rate of the increase of production can, however, be formed, as several uncertain economic factors have to be taken into account. Among these are the precise extent of demand, the limit of the inevitable fall in price with largely increased production, the cost of labour as increasing amounts are required, and the effect of changed conditions on the output of “wild” rubber and the competition of the new plantations which are being established in tropical America.
There can be little doubt that with a fall in price further uses for rubber would arise, leading to an increased demand, and among them may be mentioned its utilization as a road material. Difficulties in the supply of labour in the East may hinder the further development of the rubber-planting industry, especially at a period when a reduction in the cost of production may be the chief problem. In 1909 the average
cost of producing “plantation” rubber in Ceylon and Malaya 
Fig. 11.—PARA RUBBER PLANTATION, CEYLON.

Fig. 12.—PARA RUBBER TREES, TAPPED—CEYLON.
(Spiral and V Systems.)
From Photographs in the Collection of the Imperial Institute.
 |

|
| Fig. 13.—CEARA RUBBER TREE. | Fig. 14.—CASTILLOA RUBBER TREES. |
 |

|
| Fig. 15.—FICUS ELASTICA. | Fig. 16.—FUNTUMIA ELASTICA. |
From Photographs in the Collection of the Imperial Institute.
may be stated approximately to have been from 10d. to 1s. per ℔. The cost of collecting “wild” rubber is less easy to state with any approach to accuracy, since the cost varies in different districts of S. and Central America, but the average cost is stated not to be less than 1s. per ℔. In Africa the cost of collection is much less, but the rubber is generally of inferior quality.
The market price of commercial rubber is determined by the current price of “fine Para” from S. America. This is subject to considerable fluctuation, :and varied in 1900 to 1908 from 2s. 10d. to 5s. 9d. a ℔. As much as 6s. 9d. per ℔ was given for specially prepared “plantation Para.” Towards the latter part of 1904 the price of fine Para reached a high level and then considerably declined, reaching in 1907–8 a lower figure than had been recorded since 1900. At the beginning of 1908 the price gradually rose again to the neighbourhood of 4s. a ℔. During 1909, without any serious decline in production, the price rapidly rose, owing to extraordinary causes, to about 10s. a ℔, and in the early part of 1910 rose to over 12s. a ℔, and subsequently fell to about half this price. Having regard to the present cost of producing “plantation” rubber, and to the probability that, apart from a possible increase in the price of labour, this cost is susceptible of further reduction, it may be concluded that rubber production will continue to be profitable even should a considerable fall in market value take place.
The Principal Rubber Trees, their Cultivation, and the Preparation of Rubber.—Most commercial rubber is derived from natural supplies, from the wild rubber trees of S. and Central America, India and Africa. Each year, however, the output of “plantation” rubber will show a considerable increase, and it is to be expected that ultimately this will form the chief source of supply, unless unforeseen circumstances should arise to interfere with the development of the plantation industry, which has been vigorously started chiefly with European capital in the tropical possessions of Great Britain, France and Germany. The best rubber is now obtained from large trees, of which the following are the more important:—
1. “Para” rubber, which takes the first position in the market, is derived from species of Hevea, principally Hevea brasiliensis, of which there are enormous forests in the valleys of the Amazon and its tributaries, and also in Peru, Bolivia, Venezuela and Guiana. In Brazil alone it is stated that the rubber area amounts to at least one million sq. m. The tree has been recently planted with great success especially in Ceylon and Malaya (Plate figs. 11 and 12).
2. “Ceara” or Maniçoba rubber is derived from species of Manihot, chiefly Manihot Glaviovii, a native of S. America especially abundant in Brazil, and successfully introduced into other countries (Plate fig. 13). The latex of this tree flows less freely than that of Hevea brasiliensis, and the collection of large quantities of the latex is attended with considerable difficulty. The latex is therefore usually allowed to coagulate on the tree, as it slowly exudes from the incision. On this account it is often exported in strings or “scrap” and not usually in biscuits or balls. Partly for this reason and partly because pieces of wood and dirt are apt to be included with the scrap, the market value of Ceara rubber is usually less than that of Para. The plantations of Manihot established in E. Africa, Ceylon and S. India have, however, begun to furnish a better quality of Ceara rubber, which is often prepared in biscuit form. Other species of Manihot are also under trial, and some give promise of good results, especially M. dichotorna and M. heptaphylla.
3. The “Ule” rubber of Central America and British Honduras originates from Castilloa elastica. In S. America its natural occurrence appears to be limited to west of the Andes, but the tree is abundant in Mexico, Guatemala and Nicaragua. The rubber comes into commerce in thick strips or sheets or as “scrap.” The rubber is usually dark in colour and is often contaminated with proteid impurities derived from the latex. Ule rubber is generally inferior in strength to Para and commands a lower price. The Castilloa tree has been experimentally planted in Ceylon, the West Indies and other countries (Plate fig. 14).
Other trees occurring in America which furnish rubber of secondary commercial importance are Hancornia speciosa, yielding the Mangabeira rubber of Brazil, and species of Sapium furnishing the Colombian rubber and much of the rubber of Guiana (derived from Sapium Jenmani), which is scarcely inferior to the rubber of Para.
4. “Rambong” or Assam rubber is the produce of Ficus elastica, commonly known as the indiarubber tree and cultivated in Europe as an ornamental plant. This tree, indigenous to Asia, attains large dimensions in India, Celon and the Mala Archipelago (Plate fig. 15). It furnishes most of the rubber of, India, Sumatra and ]ava. Although intrinsically of excellent quality, Rambong rubber, owing to the careless method of collection practised 'by the natives which leads to the inclusion of much impurity, usually fetches a lower price than Para. The tree has been introduced into W. Africa and Egypt, but has not proved very successful in Africa as a rubber producer.
5. “Lagos” rubber is the produce of the African rubber tree Funtumia elastica, which is indigenous to Africa from Uganda to W. Africa (Plate fig. 16). It is known as the silk rubber tree, probably on account of the silky hairs which are attached to the seeds. The latex, which is usually coagulated by standing or by heating, is obtained from incisions in the bark of the tree. The rubber is of good quality, though, owing to the method of preparation adopted, the product is often impure and discoloured, and consequently usually brings a lower price than the best rubbers of commerce.
6. Besides the trees described above, a number of climbing plants or vines belonging to the Apocyanaceae secrete a latex which furnishes rubber of good quality. These vines are less satisfactory than trees as rubber producers, owing to the readiness with which they are injured and destroyed by careless tapping, and to the difficulty of regulating these methods in the case of vines distributed over enormous areas of forest. Of these vines the most important are the species of Landolphia which occur throughout tropical Africa, including the Sudan, Congo, Mozambique and Madagascar, the principal of which are Landolphia owariensis and L. Heudelotii, common throughout W. Africa, and L. Kirkii and L. Dawei in E. Africa. The rubber is obtained by incising the stems of the vines and coagulating the latex by exposure, by admixture with acid vegetable juices or by heating. Landolphia rubber is usually roughly prepared and in consequence commands a low price. The vines of species of Clitartdra and Carpodinus in W. Africa also furnish good rubber, as do the Forsteronia gracitis of British Guiana and Forsteronia fioribunda of Jamaica. Vines resembling Landolphias are widely distributed in Asia. Among these are species of Willughbeia and Leuconotis, from which much of the rubber exported from Borneo is derived; Parameria glandulifera, common in Siam and Borneo, and Urceola esculenta and Cryptostegia grandiflora, both common in Burma.
Among other sources from which rubber is commercially obtained may be mentioned the Guayule plant (Parthenium argentatum) of Mexico, and the “Ecanda” plant of Portuguese W. Africa, from the tuberous roots of which rubber is extracted by the natives. The “Ecanda” plant has been named Raphionacme utilis. The root rubber prepared by the natives of the Congo and the S. Sudan is extracted partly from the roots of Landolphia or from the rhizomes of Landotphia Thollortii or Carpodirtus lanceotatus. It is obtained by breaking up the roots or rhizomes in hot water and separating the rubber, and machines have now been devised for this purpose. Little is at present known of the large rubber tree of Tonkin (Bleckrodea tonkinensis), the latex of which is stated to furnish excellent rubber.
Sources of Commercial Rubber
1. Para Rubber is so named from the Para province of Brazil, from the principal town of which, also known as Para, most of the rubber is shipped. This rubber is obtained chiefly from Hevea brasiliensis, Müll. Arg., a large euphorbiaceous tree upwards of 60 ft. in height, and having trifoliate leaves, the leaflets being lanceolate and tapering at both ends (fig. 1). The trunk reaches about 8 ft. in circumference. The flowers are usually pale green. The fruit is a capsule containing three seeds rather larger than cobnuts, having a brown smooth surface figured with black patches. The seeds readily lose their vitality, and on this account need special care in transport. They should be loosely packed in dry soil or charcoal. These seeds have been examined at the Imperial Institute, and the kernels have been found to contain nearly half their weight (48%) of an oil resembling linseed oil and applicable for the same purposes. The residue or “cake” left after expression of the oil is apparently nutritious and may prove to be of value for feeding animals. There is present in the seeds, an enzyme which rapidly decomposes the oil if the seeds are crushed and kept, setting free a fatty acid and glycerin. As the seeds are very abundant, they will probably be utilized commercially as soon as the demand for planting has subsided.
In Brazil the trees are found in different districts, but flourish best on rich alluvial clay slopes by the side of rivers, where there is a certain amount of drainage, and the temperature reaches from 89° F; to 94° F. at noon and is never cooler than 73° F. at night, while rain falls during about six months and the soil and atmosphere are moist throughout the year. The genus Hevea was formerly called Siphonia, and the tree named Pao de Xerringa by the Portuguese, from the use by the Omaqua Indians of squirts or syringes made from a piece of pipe inserted in a hollow flask-shaped ball of rubber. The trees are not generally tapped until they are ten to fifteen years old, as young trees yield inferior rubber. If carefully conducted, tapping does not injure the tree. The latex is collected in the so-called dry season between June and February. The trees are tapped in the early morning when the latex is most readily obtained. To obtain the latex, deep incisions are made near the base of the tree extending up the trunk. Small shallow cups are placed below the

Fig. 1.—Hevea brasiliensis.
incisions to receive the milk, each cup being attached by sticking a piece of soft clay to the tree and pressing the cup against it. The latex, of which each tree yields only about 6 oz. in three days, has a strong ammoniacal odour, which rapidly disappears, and in consequence of the loss of ammonia the latex will not keep for longer than a day unchanged; hence when it has to be carried to a distance from the place of collection, 3% of ammonia solution is added. The latex usually furnished about 30% of rubber.
To obtain the rubber, the latex is usually treated in the following manner. A piece of wood about 3 ft. long, with a flattened end forming a kind of paddle, is dipped in the milk, or this is poured over it as evenly as possible. The milk is then carefully dried by turning the mould round and round in the smoke produced by burning wood mixed with certain oily palm nuts; those of Attalea excelsa are considered best, the smoke being confined within certain limits by the narrowness of the neck of the pot in which the nuts are heated. The creosote and other products from the smoke no doubt act antiseptically and prevent to a large extent the subsequent putrefaction of the proteids retained by the coagulated rubber. Each layer of rubber is allowed to become firm before forming another; a practised hand can make 5 or 6 ℔ in an hour. In some districts a stout stick is substituted for the paddle, on which the rubber as it coagulates is wound cylindrically. The rubber thus prepared is the finest that can be obtained. The cakes when completed are, in order to remove them from the mould, slit open with a sharp knife, which is kept wet, and are hung up to dry. The Hat rounded cakes of rubber made in this manner are known in the London market as “biscuits.” They retain about 15% of moisture. The scrapings from the tree, which contain fragments of wood, are mixed with the residues of the collecting pots and the refuse of the vessels employed, and are made up into large rounded balls, which form the inferior commercial quality called “negro head,” and often contain 25 or 35% of impurity. The yield of rubber varies, but it is stated on an average to be 10 ℔ of rubber per tree, and if carefully tapped one tree will yield this amount for many years in succession.
Plantations of Hevea brasiliensis.—Hevea brasiliensis was introduced to Ceylon and Singapore from seedlings raised at Kew from Brazilian seed, specially collected by Mr H. A. Wickham in S. America. The seedlings rapidly developed and in most places in which they were planted grew into large trees which furnished satisfactory latex when tapped in their sixth or seventh year. Ever since plantations of Hevea have been made on an increasing scale in the Straits Settlements, the Federated Malay States and in Ceylon, and at the present time rubber plantations form the principal industry in these colonies. Successful plantations of Hevea have also been established in ]ava, Sumatra and Borneo. Many of these plantations have not yet reached the productive stager-that is, the sixth or seventh year. A large number of plantations in British Malaya and Ceylon are now actively exporting increasing quantities of rubber. Hevea seedlings were also introduced into India, but did not apparently succeed except in Burma and S. India. It may be estimated that between one and two million acres of land in the different countries referred to have been already appropriated for rubber plantations. Plantations are also being formed in British, French and German possessions in W. Africa and in the Congo, also in the tropical ortions of Australia. In certain districts of British W. Africa the Hevea which has been planted promises well, especially in the Gold Coast, where good yields of latex are stated to have been obtained.
It may be useful to summarize here the experience which has been gained in the formation of plantations of Hevea and in the production of rubber.
Hevea brasiliensis as a rule flourishes to the greatest extent at low altitudes on rich soil capable of retaining moisture. The nature of the soil appears, however, to be of secondary importance, provided that it is able to hold moisture and that climatic conditions of high and even temperature with considerable rainfall and absence of wind are satisfied. Although the tree is sensitive to such conditions it appears to possess a certain capacity of adaptation which should be borne in mind. Generally a low altitude is desirable, but good results have been obtained in Ceylon in sheltered positions at elevations of 3000 ft. and over, although at higher altitudes the growth of these trees appears to be slower. In many plantations besides catch crops (cassava, sesame, ground-nuts, &c.) other crops, such as tea, coffee, cocoa and tobacco, are grown with rubber. It is improbable, except in the early stages of the rubber tree, that this procedure will succeed; the rubber will ultimately dominate the position to the detriment and ultimate extinction of the other crop, whilst the growth of the rubber tree will be retarded. A partial exception may perhaps be made in the case of cocoa, when the two plants are placed not too closely in about equal numbers. In these circumstances it appears that satisfactory results may be obtained from both crops, at any rate fora certain number of years.
The experience of planters. in general is in favour of the complete removal of weeds from a rubber plantation. This practice, which involves periodical weeding, adds considerably to the cost of maintaining plantations, and, although justified so far by results, possesses several other disadvantages. During the tropical rain; the soil is liable, to a greater or less extent, to denudation, which becomes very serious when the land slopes; and in any case, the soil is apt to become impoverished by the loss of its soluble constituents. These disadvantages are at their maximum when the rubber trees are quite young. At a later stage the shade of the large trees compensates to a considerable extent for the absence of cover on the ground. Another disadvantage of uncovered soil in a plantation of young rubber trees is that the ground under the heat of a tropical sun rapidly loses its moisture. For this reason proposals have been made to plant in the place of weeds low-growing leguminous plants, the growth of which will not only prevent impoverishment and loss of soil during the rains and conserve moisture in the heat, but will also have the effect of enriching the soil in nitrogenous constituents through the power leguminous plants possess of absorbing nitrogen from the air through nodules on their roots. Among the plants which are being tried for this purpose are various species of Crotolaria, passion-flower, and the well-known sensitive plant of the East. The success of the method cannot yet be judged, but the experiment is one which deserves very full trial.

Fig. 2.—Tapping, herring-bone system.
One of the most important subjects in connexion with rubber plantations is the method to be adopted in tapping the trees for latex. The native methods in vogue in Brazil and Mexico are primitive and often injurious to the tree. At present it cannot be said that finality has been reached on the subject of the best method, giving a good return of latex with a minimum of damage to the tree. A method at one time largely adopted was to make a series of V-shaped incisions on four sides of the tree to a height of 6 ft. from the base—that is, within the reach of an ordinary man without the need for ladder or scaffolding; the latex obtained from the upper part of the tree is said to furnish less rubber and of poor quality. The latex is collected in cups placed at the apex of each V. Other systems are the herring-bone plan of a vertical channel with lateral connecting channels about 1 ft. apart at an angle of about 45°, the latex being collected in cups placed at the base of the vertical channels (fig. 2); the spiral system, in which a series of spiral grooves are cut all round the trunk, by which means virtually the entire area of the trunk is tapped. In some instances a combination of these methods is employed. The V-system is the oldest, but is being largely superseded by the herring-bone; the spiral system is more recent and is still on trial.
Instead of the axe or large knives which frequently inflicted serious damage to the trees, special small knives and prickers are now employed so constructed as to avoid injury to the tree through making a larger incision than is necessary, and without penetrating into the wood below the laticiferous layer. It is possible to tap or prick trees daily for a number of years without apparent injury, but the practice of tapping on alternate days appears to be safer and to afford equally satisfactory if not better results. The yield of latex is at first small, but increases with successive tapipings, which appear to stimulate the local production of latex, and finally reaches a maximum.
When the bark has been removed a period of from three to four years must elapse before it is so fully renewed as to render fresh incisions possible. In the case of a tree from seven to ten years old, tapping is so arranged that by the time the last incisions on the original growth are made, the new growths on other portions are at least four years old, and ready for new incisions to be made. Too frequent tapping leads to the production of latex poor in caoutchouc, whilst tapping of trees before they are six or seven years old, and from 20–25 in. in circumference, produces inferior rubber. As a rule, an annual yield of more than 1–2 ℔ of rubber per tree must not be looked for from recent plantations, although much higher yields up to 10–15 ℔ and over per tree are recorded from S. America, and it is therefore probable that with greater experience as to the best methods of tapping and with older trees considerably larger yields may be expected from plantations in the future. An average of 150 trees to the acre (20×15 ft.) and a yield of 11/2 ℔ of rubber per annum per tree at 2s. 6d. per ℔ gives the result of £28, 2s. 6d. per acre. The cost of production may be assumed to be about 1s. per ℔, to which has to be added the expense of transport. The cost of clearing forest land and planting with rubber in geylon is estimated at about 100 Rs. per acre in the first year, and from 20–30 Rs. per acre in subsequent years until the sixth year, when the plantation would begin to be productive.
The point of next importance is the coagulation of the latex so as to produce rubber in the form and of the quality required by the manufacturer. The primitive methods of coagulation and curing practised in S. America undoubtedly are susceptible of considerable improvement, and certainly waste can be. reduced to a minimum. It is, however, important to remember that rough as these native methods are they result in the production of rubber which commands the highest price. As the removal of the impurities of the latex is one of the essential points to be aimed at, it was thought that the use of a centrifugal machine to separate the caoutchouc as a cream from the watery part of the latex would prove to be a satisfactory process. This method is said to answer well with the latex of Castilloa, but it appears to be inapplicable to the latex of Het/ea, which does not cream readily when centrifugalized.
The plan usually adopted is to collect the latex in rectangular tanks or casks. It is then coagulated by the addition of an acid liquid, acetic acid or lime juice being generally employed, and the mixture allowed to stand. The coagulated rubber separates as a mass of spongy caoutchouc. If the coagulation has been effected in shallow dishes, the rubber is obtained in a thin cake of similar shape known as a “biscuit.”
The rubber thus formed is washed and dried. The coagulated rubber separated from the watery fluid is cut up into small pieces and passed through the grooved rollers of the washing machine, from which it issues in sheets, long crinkled ribbons or “crepe,” which are then dried in hot air chambers or in a vacuum dryer, by which means the water is dissipated at a lower temperature. In order to prevent decomposition of any proteid impurity which may remain incorporated with the rubber, the freshly coagulated rubber is sometimes cured in the smoke of burning wood or a small quantity of an antiseptic such as creosote is added during coagulation.
Plantation rubber comes into commerce in the form of the crinkled ribbons known as crepe, in sheets or biscuits, and sometimes in large blocks made by compressing the crepe rubber. Block rubber is considered to possess certain advantages in securing a constant proportion of water, and in being satisfactory for transport. The best condition and form in which to export rubber cannot be regarded as settled. The probabilities are that in the end the production of a rubber as nearly as possible free from water and impurities and of constant composition will be realized as best meeting the requirements of the modern manufacturer. The need for scrupulous cleanliness in the preparation of rubber is now recognized, and the arrangements of a rubber factory in Ceylon or Malaya are comparable with those of the modern dairy.
In the present transition stage of rubber production it is necessary for the manufacturer in Europe to wash all rubber. He receives both the wild rubber contain in variable quantities of impurity and the purer plantation rubber, tie latter, however, in much smaller amount. The fact that at present washing machinery exists in all European factories and that most of the rubber received needs washing, leads to the greater purity of plantation rubber, except for special purposes, being generally discounted by the manufacturer. As soon as the output of plantation rubber of constant composition has reached much larger dimensions it is probable that the manufacturer will be able to dispense with washing. This will operate to the advantage of plantation rubber and against the wild rubber, so long as the latter is not exported in a purer condition.
So far the Hevea, plantations in Ceylon and the East have not been seriously troubled by insect or fungoid pests, and those which have occurred have succumbed to proper treatment. The most serious trouble has been occasioned in the Malay States by a white thread-like fungus (Fomes semitostus) which attacks the roots of the Hevea tree and eventually kills it. The development of this fungus is greatly promoted by the presence of decaying stumps and wood in the plantation. Vigorous measures are now taken in many plantations to remove all old wood and to extract stumps of old trees, which in the first instance it was considered unnecessary to remove.
2. Manihot Glaziovii belonging to the Euphorbiaceae is the tree of N.E. Brazil which furnishes Ceara or Maniçoba rubber (fig. 3). It is closely related to the Manioc, cassava or tapioca plant (Manihot utilissima) which it resembles when young and exhibits a similar tuberous root system. The tree grows well on dry and rocky soil without rain for a considerable period of the year, and flourishes at high altitudes up to about 4000 ft. It is therefore adapted for conditions which are unsuitable for Hevea. The tree grows about 30 ft. high, with a rounded head of foliage, and greyish-green 3 to 7-lobed palmate leaves, somewhat resembling the leaves of the castor-oil plant in shape and size. The seeds (fig. 3), which are abundant and retain their vitality well, have a hard thick coat. The seeds take a year to germinate, unless the edges near the end bearing the carbuncular projecting are rasped off. Cuttings, if they have a-single bud, strike readily.

Fig. 3.—Manihot Glaziovii. 1, branch with flowers; 2, fruit; 3, seed.
The trees are tapped when they are about five years old. The mode of collecting the rubber is as follows. After brushing away the loose stones and dirt from the root of the tree by means of a handful of twigs, the collector lays down large leaves for the latex to drop upon. He then slices off the outer layer of the bark to the height of 4 or 5 ft. The latex, which exudes slowly and in many tortuous courses, some of it ultimately falling on the ground, is allowed to remain on the tree for several days, until it becomes dry and solid, when it is pulled off in strings, which are either rolled up into balls or put into bags in loose masses, in which form it enters commerce under the name of Ceara “scrap.” Ceara rubber is also exported in the form of lumps and cakes. The annual yield of rubber is rather more than 1 ℔ per tree. The latex coagulates readily, especially if churned or if diluted with water, when a purer rubber is obtained.
The Manihot tree has been widely introduced into other countries, and appears to succeed wherever the rainfall is not excessive. ' In Ceylon and in some parts of India, especially in Madras, it has succeeded well. In W. Africa the tree flourishes, but it is under trial as a rubber producer. The Manihot tree also promises well in E. Africa, Nyasaland and the Mozambique. The pure Ceara rubber, as for example the “biscuits” prepared in Ceylon, is of excellent quality, scarcely if at all inferior to Para. That derived from Brazil, however, is generally inferior, being mixed with wood and dirt. The cultivation and collection of, the rubber being troublesome, it is unlikely to be attended to in those countries in which Hevea is successful.
3. The source of “Ule” rubber exported from Central America, and of the “Caucho” rubber of Peru is Castilloa elastica, Cerv., a lofty tree, N. O. Urticaceae, with a trunk 3 ft. or more in diameter, and large hairy oblong lanceolate leaves often 18 in. long, and 7 in. wide (fig. 4). The tree grows most abundantly in a sporadic manner in the dense moist forests of the basin of the Rio San Juan, where the rain falls for nine months in the year. It prefers rich fertile soil on the banks of watercourses, but does not flourish in swamps. It is found also in Costa Rica, Guatemala, Honduras, Mexico, Cuba and Hayti, and in Panama with another species of Castilloa, and on the W. coast of S. America down to the slopes of Chimborazo; the Cordilleras of the Andes separating the Castilloas from the Heveas of Brazil.
In Nicaragua the latex is collected in April, when the old leaves begin to fall and the new ones are appearing, during which time the latex is richest. The tree is tapped either in the same manner as the Hevea, or by encircling the tree with a simple spiral cut at an inclination of 45°, or by two parallel spirals if the tree be large. At the bottom of the spiral an iron spout about 4 in. long is driven into the tree, and the milk is received in iron pails. A tree 20 to 30 ft. high to its first branches, and about 4 ft. in diameter, is expected to yield annually 20 gallons of milk, each gallon giving about 2 ℔ of rubber. In the evening the milk is strained through a wire sieve and transferred to barrels. The milk, which is acid, is coagulated by the addition of the alkaline juice of the “achete” plant, or of another plant called “coasso.” The strained Juice of either of these plants, obtained by bruising the moistened herb and subsequent expression, is added to the milk in the proportion of about 1 pint to the gallon. In British Honduras an alkaline decoction prepared from the Moon plant (Calonictyon speciosum) is used for the same purpose. If these plants are not procurable, two parts of water are added to one of the milk, and the mixture allowed to stand for twelve hours. The coagulum is next flattened out by a wooden or iron roller to get rid of the cavities containing watery liquid, and the sheets are then hung up for fourteen days to dry, when they weigh about 2 ℔, the sheets being usually 1/2 to 1/8 in. thick and 20 in. in diameter. When coagulated in water, the mass is placed in vats in the ground and allowed to dry, this taking place in about a fortnight. It is then rolled into balls. That which dries on the incisions in the tree is called “bola” or “burucha,” and is said to be highly prized in New York. The loss of Nicaragua rubber in drying is estimated at 15%. It is exported chiefly from San Juan del Norte, or Grey Town, and the larger proportion goes to the United States. The Castilloa tree appears to be suitable for cultivation only in districts where the Para rubber would grow equally well. The tree is ready for tapping at about the same age as Hevea and the average yield of rubber is about the same. Since the latex “creams” readily the rubber can be separated from the latex by centrifugalizing, and its quality and market value thus enhanced. Much of the native Castilloa rubber is of inferior quality. The tree has been introduced into S. India, Ceylon and the W. Indies, where it has succeeded well, especially in Trinidad and Tobago. It is also under trial in E. and W. Africa and Nyasaland. Several other species of Castilloa than C. elastica are known to furnish rubber, but little has been recorded as to their advantages.
4. Funtumia elastica (formerly known as Kickxia or Kixia elastica) is the W. African (Ire or Irai or Lagos) rubber tree, which belongs to the Apocynaceae, a natural order which includes the Landolphia vines as well as other rubber producers. It is a large forest tree of upright habit extending to 60 or 70 ft. in height and 3 to 4 ft. in diameter. The bright green, glabrous leaves are broad and oblong, about 6 in. in length (see fig. 5). The flowers are yellow, and the seeds enclosed in a pod are long and thin with numerous long silky fibres attached to them, which enable the seeds to be readily carried by the wind. The trees are common throughout the central regions of E. and W. Africa (from Uganda to Sierra Leone). The botanical name is taken from a W. African native name for a rubber tree—“Funtum.”
Many of the trees in the accessible forests of W. Africa have been destroyed by over-tapping and felling. Plantations of Funlumia have been established in several districts, including the Gold Coast and S. Nigeria. The trees are tapped on the “herring-bone” plan and the milk collected in vessels at the base. This is then poured into the hollowed-out trunk of a tree, where it is allowed to stand covered with palm leaves for about a fortnight. The watery portion of the latex soaks into the trunk, and the soft spongy rubber which remains is kneaded and pressed into lumps or balls.

Fig. 4.—Castilloa elastica. 1, leaf; 2, twig with male flowers; 3, twig with female flowers; 4, seed; 4, nat. size.
In some districts the collected milk is heated alone or diluted with water, to coagulate the rubber, but if heated alone an inferior rubber is apt to result owing to overheating.
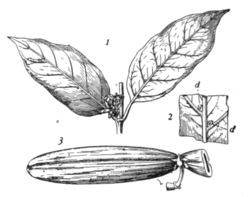
Fig. 5.—Funtumia elastica (Lagos rubber). 1, twig with flowers; 2, part of under side of leaf showing somatia at d d (about nat. size); 3, fruit (about 1/3 nat. size).
The Funtumia latex can also be coagulated by the astringent infusion of Bauhinia leaves or by exposing it in shallow dishes, when the liquid “creams.” The yield of rubber is stated as a rule to be less than that of Para. The rubber, if properly prepared, is of excellent quality, and the tree deserves further attention, especially in those regions of W. Africa which are unsuited to Hevea.
Funtumia africana furnishes a very inferior rubber, which is highly resinous.
5. Ficus elastica is the tree which produces Rambong or Assam rubber. It is well known in Europe as a small ornamental tree, but in the tropics it attains very large dimensions, and develops a system of branching roots which act as buttresses to the large trunk (see fig. 6). It is a native of India, Burma and the Malay Archipelago and is most abundant, in those regions in which the climate is distinctly humid, and subject to this condition the tree flourishes at high altitudes. In Assam and in upper Burma there are extensive forests of Ficus elastica, but to a large extent the trees have been damaged by careless tapping. Large plantations have been formed by the Government of India both in Assam and Bengal, but most of the rubber exported is still obtained from the forest trees.

Fig. 6.—Ficus elastica. 1, twig; 2, section of inflorescence.
It has been found that although the tree grows well in many different countries and different localities, it only furnishes a satisfactory yield of rubber in mountainous districts, such as those of Assam and certain parts of Ceylon and Java. The trees. are tapped when about ten years old, and as a rule annually furnish from 5–10 ℔ of rubber per tree. The latex flows fairly well, but is usually allowed to dry on the tree.
The rubber, if of good quality, sells at prices only slightly inferior to that of Para. When the plantations of Ficus in India are in full bearing it is possible that this tree may attract more attention, since the plantation rubber is likely to be of superior quality owing to the greater care taken in its preparation. It seems at present doubtful, however, whether the establishment of plantations of Ficus will be profitable under ordinary conditions in India.
In addition to the trees described above there are numerous plants of some importance as rubber producers. Among these may be mentioned the Landolphia vines, which are still the chief source of African rubber. The vines grow upon forest trees, and the stems are periodically tapped. There are numerous species of these climbing plants, of which the most important as furnishing good rubber are Landolphia owariensis (see fig. 7), which occurs throughout W. Africa and the Sudan, Landolphia Heudelotii of W. Africa, and Landolphia Kirkii and L. Dawei, which are found in the forests of E. Africa. Other species of Landolphia, including Landolphia florida, abundant in both E. and W. Africa, furnish rubber of inferior quality.

Fig. 7.—Landolphia owariensis. 1, twig with flowers; 2, fruit.
Among other shrubs and vines which yield rubber of fair quality may be mentioned Willughbeia edulis and Urceola elastica and Parameria glandulifera, which occur in Burma and Malaya.
The Sapiums of Colombia and Guiana are large trees resembling Hevea, and certain species furnish good rubber, especially the Sapium Jenmani of Guiana. Most of the native Sapiums have been destroyed by reckless tapping, and the merits of this genus have been somewhat overlooked and deserve reinvestigation. The same applies to certain species of Hevea, other than H. brasiliensis, which are known to produce good rubber in tropical America.
Pernambuco or Mangabeira rubber is obtained from Hancornia speciosa, Gom., an apocynaceous tree common on the S. American plateau in Brazil from Pernambuco to Rio de Janeiro, at a hei ht of 3000 to 5000 ft. above the sea. It is about the size of an ordinary apple tree, with small leaves like the willow, and a drooping habit like a weeping birch, and has an edible fruit like a yellow plum called “mangaba,” for which, rather than for the rubber, the tree is cultivated in some districts. Only a small quantity of this rubber comes to England, and it is not much valued, being a “wet” rubber. It is produced in “biscuits” or “sheets.” The caoutchouc is collected in the following manner: about eight oblique cuts are made all round the trunk, but only through the bark, and a tin cup is fastened at the bottom of each incision by means of a piece of soft clay. The cups when full are poured into a larger vessel, and solution of alum is added to coagulate the latex. In two or three minutes coagulation takes place, and the rubber is then exposed to the air on sticks, and allowed to drain for eight days. About thirty days afterwards it is sent to market. Pernambuco rubber, as is the case with most rubbers coagulated by saline solutions, contains a large quantity of water. The tree has been planted in other countries, but has so far not received much attention. It will grow on a dry sandy soil, dislikes much moisture, and needs no shade.
Forsteronia gracilis of Guiana is a climbing plant which also belongs to the Apocynaceae. Like the Forsteronia floribunda of Jamaica it yields rubber of good quality. Ficus Vogelii of W. Africa yields rubber of variable quality. The production of rubber by this tree merits further investigation, as it grows readily in nearly every district of W. Africa and the Sudan.
Specimens of the best known and of many of the lesser known rubbers are included in the Colonial and Indian Collections and Sample Rooms of the Imperial Institute, and many of the authentic specimens have been chemically and technically examined in the Scientific and Technical Department of the Institute and commercially valued. Reports on many of the lesser known rubbers have been published in the Bulletin of the Imperial Institute.
Chemistry of Rubber.
Rubber is chiefly composed of the soft, solid, elastic substance known as caoutchouc. It is usually assumed that this substance is present as such in the latex. The globules in the' latex, however, consist more probably of a distinct liquid substance which readily changes into the solid caoutchouc. The coagulation of the latex often originates with the “curding” of the proteids present, and this alteration in the proteid leads to the solidification of the globules into caoutchouc. The latter, however, is probably a distinct effect. Under certain conditions, as when latex is allowed to stand or is centrifugalized, a cream is obtained consisting of the liquid globules, which may be washed free from proteid without change, but, either by mechanical agitation or by the addition of acid or other chemical agent, the liquid gradually solidifies to a mass of solid caoutchouc. The phenomenon therefore resembles the change known to the chemist as polymerization, by which through molecular aggregation a liquid may pass into a solid without change in its empirical composition. The effect may, however, also be due to chemical change known as condensation, and be accompanied by the elimination of the elements of water. So far the chemical nature of the liquid globules of the latex is unknown, and the exact character of the change into solid caoutchouc remains to be determined. The watery liquid known as rubber milk or latex is an emulsion consisting chiefly of a weak watery solution of proteids, carbohydrates and salts holding the liquid globules in suspension. In connexion with the production of rubber the most important factor is the proportion of caoutchouc it contains. In a good rubber this ranges from 70–90% and over. The proportion and nature of the proteids or albuminous materials varies considerably in different latices. The proteids should be as far as possible removed during the preparation of the rubber, as these substances are chiefly responsible or the objectionable smell and colour of “native” rubbers, and their presence leads to subsequent change in the commercial material. All crude rubber contains more or less proteid, and in the opinion of some technical experts its presence even affords strength to the material, but this cannot be accepted as proved. The dissolved salts (potassium, sodium, ammonium, calcium, magnesium, &c.) of the latex are generally nearly entirely absent from the well prepared rubber. Of considerable importance to the value of the rubber is the absence of the resinous constituents which are present in greater or smaller proportion in all latices. The presence of more than a small percentage of resin in the latex leads to the production of rubber containing much resin, which seriously depreciates its commercial value for most purposes. The percentage of resin in a good rubber should be as small as possible, and should in any case be less than 10%. There is no feasible method at present known of preventing the inclusion of the resin of the latex with the rubber during coagulation, and although the separation of the resin from the solid caoutchouc by means of solvents is possible, it is not practicable or profitable commercially. A complete examination of a series of different latices has shown that, in many cases, e.g. Hevea and Castilloa, the resin is present in large proportion in the latex derived from young trees, and diminishes in amount as the tree ages. This is one reason why young trees should not be tapped. The composition of latex and of typical rubbers is given below:-
| Para Latex (Ceylon). |
Para Rubber (Ceylon). |
Ceara Rubber (Ceylon). |
Castilloa Rubber (Ceylon). |
Ficus Elastica (Bengal). |
Landolphia Kirkii (E. Africa). | ||
| % | % | % | % | % | % | ||
| Water | 55.15 | Caoutchouc | 94.6 | 76.25 | 86.19 | 84.3 | 80.1 |
| Caoutchouc | 41.29 | Resin | 2.66 | 10.04 | 12.42 | 11.8 | 6.9 |
| Proteids | 2.18 | Proteids | 1.75 | 8.05 | 0.87 | . . | 0.3 |
| Sugar, etc. | 0.36 | Ash | 0.14 | 2.46 | 0.20 | 0.8 | 7.7 |
| Ash (salts) | 0.41 | Moisture | 0.85 | 3.20 | 0.32 | 0.8 | . . |
The chemical analysis of crude rubber is an important guide to its value. At present, however, the methods of analysis usually employed are not sufficiently delicate to afford all the necessary information as to the intrinsic value of the higher grades of rubber, and do not go much beyond the exclusion of inferior rubber. The tests of the physical properties of crude rubber usually applied to determine its value in the market are also very rough and cannot be relied upon. The development of the rubber industry has now reached a stage at which more exact methods of determining the chemical composition and physical properties (strength and elasticity) of rubber are required. At present the caoutchouc present in crude rubber is usually estimated indirectly, and it is possible that what generally passes as caoutchouc may be in some instances a mixture of similar chemical substances, which if separated would be found to differ in those physical properties on which the technical value of rubber depends.
It is already certain that some commercial rubbers contain a variable proportion of a substance of the nature of caoutchouc, but having different properties.
True caoutchouc, the principal constituent of all rubbers, is probably essentially one and the same substance, from whatever botanical source it may have been derived. This is an elastic solid, almost transparent in thin sheets, composed entirely of carbon and hydrogen, the empirical composition of which is represented by the formula C5H8. It thus possesses the same composition as the hydrocarbon of gutta-percha and as that of oil of turpentine and other terpenes which are the chief components of essential oils. The properties of caoutchouc clearly show, however, that its actual molecular structure is considerably more complex than is represented by the empirical formula, and that it is to be regarded as the polymer of a terpene or similar hydrocarbon and composed of a cluster of at least ten or twenty molecules of the formula C5H8. When solid caoutchouc is strongly heated it breaks down, without change in its ultimate composition, into a number of simpler liquid hydrocarbons of the terpene class (dipentene, di-isoprene, isoprene, &c.), of which one, isoprene (C5H8), is of simpler structure than oil of turpentine (C10H16), from which it can also be obtained by the action of an intense heat.
When this volatile liquid hydrocarbon (isoprene) is allowed to stand for some time in a closed bottle, it gradually passes into a substance having the principal properties of natural caoutchouc. The same change of isoprene into caoutchouc may also be effected by the action of certain chemical agents. It may therefore be said that caoutchouc has been already artificially or synthetically prepared, and the possibility of producing synthetic rubber cheaply on a commercial scale remains the only problem. At present the change of isoprene into caoutchouc is mainly of scientific interest in indicating possibilities with regard to the conversion of the liquid globules of the latex into rubber and to the formation of rubber by plants. The exact chemical nature of caoutchouc is, however, not determined, and recent researches point to the view that its molecular structure may even be somewhat different from that of the terpenes.
The exact manner in which isoprene passes into caoutchouc is also not understood. These problems are, however, certain to be solved in the near future, and then probably caoutchouc may be formed in other ways than from isoprene.
The question as to whether synthetic rubber will ever be produced cheaply on a commercial scale is therefore the important one for those who are largely interested in the rubber-planting industry. No definite answer can be given to this question at the present time. Its settlement will depend in part on the cost of producing rubber from plants, which from their point of view it is to the interests of planters to reduce as far as possible. There are many substances produced by plants which can be synthetically prepared by chemical means, but, as with quinine, the process involved is too costly to enable the synthetic product to compete with the natural product. The chief properties of caoutchouc and its employment for technical purposes may now be considered.
Caoutchouc is not dissolved by water or alcohol, and is not affected except by the strongest acids. Alkalis have little effect on it under ordinary circumstances, although prolonged contact with ammonia results in a partial change. The best solvents for rubber are carbon bi sulphide, benzol and mineral naphtha, carbon tetrachloride and chloroform. These liquids, either alone or mixed, are employed in making the rubber solutions used for technical purposes. Vegetable and other oils rapidly penetrate caoutchouc and lead to deterioration of its properties. Sulphur when warmed with caoutchouc combines with it, and on this fact the vulcanization of rubber depends, and also the production, with an excess of sulphur, of the hard black material known as vulcanise or ebonite. Caoutchouc is a soft elastic resilient solid. In this respect it differs from gutta-percha, which, like caoutchouc, is derived from the latices of certain plants. The technical value of caoutchouc chiefly depends on the extent to which it is capable of being stretched without breaking, and the extent to which it at once returns to its original dimensions. Caoutchouc is a bad conductor of heat and electricity, and alone or mixed with other materials is employed as an electrical insulator.
When caoutchouc is heated slightly above the temperature of boiling water it becomes softer and loses much of its elasticity, which, however, it recover es on cooling. At about 150°-200° C. caoutchouc melts, forming a viscous liquid which does not solidify on cooling. This viscous liquid is present in small proportion in some commercial rubbers owing to overheating during their preparation. It appears to be the principal cause of stickiness or the tacky ” condition of some rubbers, which considerably depreciates their commercial value. There is some evidence that “ tackiness ” may be induced by a kind of fermentation which takes place in crude rubber.
At higher temperatures the viscous liquid suffers decomposition with the formation of various liquid hydrocarbons, principally members of the terpene series. Similar products are also formed by heating gutta-percha which closely resembles caoutchouc in its chemical structure.
Rubber slowly absorbs oxygen when exposed to air and light, the absorption of oxygen being accompanied by a gradual change in the characteristic properties of rubber, and ultimately to the production of a hard, inelastic, brittle substance containing oxygen. Ozone at once attacks rubber, rapidly destroying it. If ozone is passed into a solution of rubber in chloroform the caoutchouc combines with a molecule of ozone forming a compound of the empirical composition C5H8O3. When this compound is acted on by water, hydrogen peroxide and levulinic aldehyde are formed, the aldehyde being subsequently oxidized by the hydrogen peroxide, forming levulinic acid. The hydrocarbon of gutta-percha yields similar results and is therefore closely related to caoutchouc. The study of the action of ozone on caoutchouc has thrown new light on the complex question of the chemical structure of this substance, and discloses relationships with the sugars and other carbohydrates from certain of which levulinic acid is obtained by oxidation.
Caoutchouc, like other “unsaturated” molecules, forms compounds with chlorine, bromine, iodine and sulphur.
Commercial Treatment of Rubber.
In the industrial working of indiarubber, the various impurities present in the crude “ wild " rubber (bark, dirt and the principal impurities derived from the latex, except resin) are removed by the following process: The lumps of crude caoutchouc are first softened by the prolonged action of hot water, and then cut into slices by means of a sharp knife—generally by hand, as thus any large stones or other foreign substances can be removed. The softened slices are now repeatedly passed between grooved rollers, known

Fig. 8.—Roller of Washing Machine.
as washing rollers (fig. 8), a supply of hot or cold water being made to flow over them. Solid impurities speedily become crushed, and are carried away by the water, while the rubber takes the form of an irregular sheet perforated by numerous holes. The loss on washing ranges from 10–15 % with “fine Para” to 40 % with other “wild” rubbers. In the future this washing of “wild” rubber may be conducted in the tropics, thus furnishing the manufacturer with rubber which, like “plantation” rubber, need not be subjected to this process in the factory. The washed product contains in its pores a notable proportion of water, which is removed by hanging the rubber for some days in a warm room. It is now ready either for incorporation with sulphur and other materials, or for agglomeration into solid masses by means of the masticating machine—an apparatus which consists of a strong cylindrical cast-iron casing, inside which there revolves a metal cylinder with a fiuted or corrugated surface. Some of the rubber having been placed in the annular space between the inner cylinder and the outer casing, the former is made to revolve; and the continued kneading action to which the rubber is subjected works it into a solid mass, something like a gigantic sausage. Before commencing the mastication it is generally necessary to warm the apparatus by means of steam; but as the operation proceeds the heat produced requires to be moderated by streams of cold water flowing through channels provided for the purpose. The inner cylinder is generally placed somewhat eccentrically in the outer casing, in order to render the kneading more perfect than would otherwise be the case.
To convert the masticated rubber into rectangular blocks, it is first softened by heat, and then forced into iron boxes or moulds. The blocks are cut into thin sheets by means of a sharp knife, which is caused to move to and fro about two thousand times per minute, the knife being kept moistened with water, and the block fed up to it by mechanical means. Cut sheets are largely used for the fabrication of certain classes of rubber goods-these being made by cementing the sheets together with a solution of rubber in' naphtha or benzol. Most articles made of cut sheet rubber would, however, be of very limited utility were they not hardened or vulcanized by the action of sulphur or some compound of that element. After vulcanization, rubber is no longer softened by a moderate heat, a temperature of 160° C. scarcely affecting it, nor is it rendered rigid by cold, and the ordinary solvents fail to dissolve it. It must, however, be distinctly understood that it is not the mere admixture but the actual combination of sulphur with indiarubber that causes vulcanization. If an article made of cut sheet be immersed for a few minutes in a bath of melted sulphur, maintained at a temperature of 120° C., the rubber absorbs about one-tenth of its weight of that element, and, although somewhat yellowish in colour from the presence of free sulphur, it is still unvulcanized, and unaltered as regards general properties. If, however, it be now subjected for an hour or so to a temperature of 140° C., a combination occurs, and vulcanized caoutchouc is the result. When a manufactured article has been saturated with sulphur in the melted sulphur bath, the heat necessary for vulcanization may be obtained either by high pressure steam, by heated glycerin, or by immersion in a sulphur bath heated to about 140° C. In this last case absorption of the sulphur and its intimate combination with the rubber occur simultaneously. Cut sheets, or articles made from them, may be saturated by being laid in powdered sulphur maintained for some hours at about 110° C. Sheets sulphured in this way can be made up into articles and joined together either by warming the parts to be united, or by means of indiarubber solution; after which the true vulcanization, or “curing,” as it is termed, can be brought about in the usual way.
Another method of vulcanizing articles made from cut sheet rubber consists in exposing them to the action of chloride of sulphur. Either they are placed in a leaden cupboard into which the vapour is introduced, or they are dipped for a few seconds in a mixture of one part of chloride of sulphur and forty parts of carbon disulphide or purified light petroleum. Vulcanization takes place in this instance without the action of heat; but it is usual to subject the goods for a short time to a temperature of 40° C. after their removal from the solution, in order to drive off the liquid which has been absorbed, and to ensure a sufficient action of the chloride of sulphur. Treatment with a warm alkaline solution is afterwards advisable, in order to remove traces of hydrochloric acid generated during the process. Another very excellent method of vulcanizing cut sheet goods consists in placing them in a solution of the poly sulphides of calcium at a temperature of 140° C. Rubber employed for the manufacture of cut sheets is often coloured by such pigments as vermilion, oxide of chromium, ultramarine, orpiment, antimony, lamp black, or oxide of zinc, incorporation being effected either by means of the masticator or by a pair of rollers heated internally by steam, and so geared as to move in contrary directions at unequal speed (fig. 9).

Fig. 9.—The Mixing Rollers.
Most of the rubber now manufactured is not combined with sulphur when in the form of sheets, but is mechanically incorporated with about one-tenth of its weight of that substance by means of the mixing rollers—any required pigment or other matter, such as whiting or barium sulphate, being added. The mixed rubber thus obtained is readily softened by heat, and can be very easily worked into any desired form or rolled into sheets by an apparatus known as the calendering machine. Vulcanization is then ensured by exposure for half an hour or more to a temperature of 135°–150° C., usually in closed iron vessels into which high-pressure steam is admitted (fig. 10).

Fig. 10.—A Vulcanizer.
Tubes are generally made up around mandrels and allowed throughout the curing to remain imbedded pulverized French chalk, which affords a useful support for many articles that tend to lose their shape during the process. Of late years a considerable amount of seamless tubing has been made much in the same way as lead piping by forcing the mixed rubber through a die and curing as above. The calendered sheets are generally cured between folds of wet cloth, the markings of which they retain; and hollow articles, such as playing balls or injection bottles, are vulcanized in iron or brass moulds, tinned inside and very slightly greased. Before it is put in, the article is roughly put together, and the expansion of the included air forces the rubber into contact with the internal surface of the mould, or a little carbonate of ammonia is enclosed. Belting intended for driving machinery is built up of canvas which has been thoroughly friction ed with the soft mixed rubber, and is cured by placing it in a kind of press kept by means of steam at a dry heat of about 140° C. Packing for the stuffing boxes of steam engines is similarly prepared from strips of rubber and friction ed canvas, as also are the so-called insertion sheets, in which layers of rubber alternate with canvas or even wire gauze. Indiarubber stereotypes are now extensively made use of as hand stamps, and attempts have been made to introduce them for press and machine printing. A plaster cast of the type is, when dry, saturated with shellac varnish and redried. Rubber mixed in the usual way with about 10% of sulphur is now softened by heat, forced into the mould, and retained there by pressure during the operation of curing, which is usually effected in an iron box heated over a gas burner to 140° C.
The ordinary Macintosh or waterproof cloth is prepared by spreading on the textile fabric layer after layer of indiarubber paste or solution made with benzol or coal-naphtha. If cotton or linen is used, it is usual to incorporate sulphur with the paste, and to effect vulcanization by steam heat; but, when silk or wool is employed, no sulphur is added to the paste, the dried coating of rubber being merely brought into momentary contact with the mixture of chloride of sulphur and carbon disulphide already mentioned. Double texture goods are made by uniting the rubber surfaces of two pieces of the coated material. Air goods, such as cushions, beds, gas bags, and so forth, are made of textile fabrics which have been coated with mixed rubber either by the spreading process above described, or by means of heated rollers, the curing being then effected by steam heat. The manufacture of overshoes and fishing boots is an analogous process, only the canvas base is more thickly coated with a highly pigmented rubber of low quality. The articles are first fashioned by joining the soft material; they are then varnished, and afterwards cured in ovens heated to about 135° C. The line vulcanized “spread sheets” are made by spreading layers of indiarubber solution, already charged with the requisite proportion of sulphur, on a textile base previously prepared with a mixture of paste, glue and treacle. Vulcanization is then effected by steam heat, and, the preparation on the cloth being softened by water, the sheet of rubber is readily removed. The required thickness of the spread sheet is very often secured by the rubber-faced surfaces of two cloths being united before curing. The threads used in making elastic webbing are usually cut from spread sheets. The manufacture of springs, valves and washers does not require any very special notice, these articles being generally fashioned out of mixed rubber, and vulcanized either in moulds or in powdered French chalk. Rollers are made to adhere to their metal spindles by the intervention of a layer of ebonite, and after vulcanization they are turned. In order to make spongy or porous rubber, some material is incorporated which will give off gas or vapour at the vulcanizing temperature,—such as carbonate of ammonia, crystallized alum, and finely ground damp sawdust. Uncombined sulphur is injurious, and often leads to the decay of vulcanized goods, but an excess of sulphur is generally required in order to ensure perfect vulcanization. Sometimes the excess is partially removed by boiling the finished goods with a solution of caustic soda, or some other solvent of sulphur. In other cases the injurious effects of free sulphur are obviated by using instead of it a metallic sulphide, - generally the orange sulphide of antimony; but, for the best results, it is necessary that this should contain from 20 to 30 % of uncombined sulphur.
It will thus be seen that for nearly all practical purposes, including tires, vulcanized rubber mixed with mineral matter is employed. Such articles contain varying proportions of rubber (12–60%), about 1–2 % of combined sulphur, and from 25–70 % of mineral matter. Vulcanized rubber is also now largely used as an electrical insulator for the construction of cables, &c., instead of gutta-percha.
When the vulcanization of rubber is carried too far, from the presence of a very large proportion of sulphur and an unduly long action of heat, the caoutchouc becomes hard, horn-like, and often black, Rubber hardened by over-vulcanization is largely manufactured under the name of ebonite or vulcanise. It is usually made by incorporating about 40% of sulphur with purified Borneo rubber by means of the usua mixing rollers, shaping the required articles out of the mass thus obtained, and heating for six, eight or ten hours to from 135° to 150°. Ebonite takes a fine polish, and is valuable to the electrician on account of its insulating properties, and to the chemist and photographer because vessels made of it are unaffected by most chemical reagents. A kind of vulcanise which contains a large proportion of vermilion or other mineral pigment is used, under the name of dental rubber, for making artificial gums and supports for artificial teeth.
Literature.—Henri Jumelle, Les Plantes à caoutchouc et à gutta (Paris, 1903); Dr O. Warburg, Les Plantes à caoutchouc et leur culture (Paris, 1902; French translation by J. Vilbouchevitch); Herbert Wright, Hevea brasiliensis or Para Rubber (Colombo, 1908); Rubber in the East: the official account of the Ceylon Rubber Exhibition, 1906, edited by J. C. Willis, M. Kelway Bamber and E. B. Denham (Colombo, 1906); Yves Henry, Le Caoutchouc dans l’Afrique occidental française (Paris, 1906); E. de Wildeman and L. Gentil, Lianes caoutchoulifères de l’Etat Independant du Congo (Brussels, 1904); C. O. Weber, The Chemistry of Indiarubber (London, 1902); Selected papers from the Kew Bulletin, iii. “Rubber” (London, 1906); Kew Bulletin, 1906–9; Bulletin of the Imperial Institute, 1903–9. (T. W. R. D.)
