1911 Encyclopædia Britannica/Solution
SOLUTION (from Lat. solvere, to loosen, dissolve). When a solid such as salt or sugar dissolves in contact with water to form a uniform substance from which the components may be regained by evaporation the substance is called a solution. Gases too dissolve in liquids, while mixtures of various liquids show similar properties. Certain solids also consist of two or more components which are united so as to show' similar effects. All these cases of solution are to be distinguished from chemical compounds on the one hand, and from simple mixtures on the other. When a substance contains its components in definite proportions which can only change, if at all, by sudden steps, it may be classed as a chemical compound. When the relative quantities of the components can vary continuously within certain limits, the substance is either a solution or a mixture. The distinction between these two classes is not sharp; though when the properties of the resultant are sensibly the sum of those of the pure components, as is nearly the case for a complex gas such as air, it is usual to class it as a mixture. When the properties of the resultant substance are different from those of the components and it is not a chemical compound we define it as a solution.
Historical.—Solutions were not distinguished from definite chemical compounds till John Dalton discovered the laws of definite and multiple proportions, but many earlier observations on the solubility of solids in water and the density of the resulting solutions had been made. As early as 1788 Sir Charles Blagden (1748–1820) made measurements of the freezing points of salt solutions, and showed that the depression of freezing point was roughly proportional to the amount of salt dissolved. About 1850 Thomas Graham published his famous experiments on diffusion, both with and without a separating membrane. In 1867 botanical investigations by M. Traube, and in 1877 others by W. Pfeffer, made known the phenomena of the osmotic pressure which is set up by the passage of solvent through a membrane impermeable to the dissolved substance or solute. The importance of these experiments from the physical point of view was recognized by J. H. van’t Hoff in 1885, who showed that Pfeffer’s results indicated that osmotic pressure of a dilute solution conformed to the well-known laws of gas pressure, and had the same absolute value as the same number of molecules would exert as a gas filling a space equal to the value of the solvent. The conception of a semi-permeable membrane, permeable to the solvent only, was used by van’t Hoff as a means of applying the principles of thermodynamics to the theory of solution.
Another method of applying the same principles is due to J. Willard Gibbs, who considered the whole problem of physical and chemical equilibrium in papers published in 1877, though the application of his principles only began to make extensive progress about twenty years after the publication of his purely theoretical investigations. The phenomena of solution and of vapour pressure constitute cases of equilibrium, and conform to the laws deduced by Gibbs, which thus yield a valuable method of investigating and classifying the equilibria of solutions.
Solubility.—Some pairs of liquids are soluble in each other in all proportions, but, in general, when dealing with solutions of solids or gases in liquids, a definite limit is reached to the amount which will go into solution when the liquid is in contact with excess of the solid or gas. This limit depends on the nature of the two components, on the temperature and on the pressure. When the limit is reached the solution is said to be saturated, and the system is in equilibrium. If the solution of a solid more soluble when hot be cooled below the saturation point, the whole of the solid sometimes remains in solution. The liquid is then said to be supersaturated. But here the conditions are different owing to the absence of solid. If a crystal bf the solid be added, the condition of supersaturation is destroyed, and the ordinary equilibrium of saturation is reached by precipitation of solid from solution.
The quantity of substance, or solute, which a given quantity of liquid or solvent will dissolve in presence of excess of the solute measures the solubility of the solute in the given solvent in the conditions of temperature and pressure. The solubilities of solids may be expressed in terms of the mass of solute which will dissolve in 100 grammes of water.
The following may be taken as examples:—
| Solute. | Chemical Constitution of the Solid. | Solubility | ||
| at 0° C. | at 20° C. | at 100°C. | ||
| Sodium chloride | NaCl | 357 | 36.0 | 39.8 |
| Potassium nitrate | KNO3 | 13.3 | 31.2 | 247.0 |
| Barium chloride | BaCl2 | 30.9 | 35.7 | 58.8 |
| Copper sulphate | CuS04 | 15.5 | 22.0 | 73.5 |
| Calcium carbonate | CaC03 | 0.0018 | — | 0.0018 |
| Silver nitrate | AgN03 | 121.9 | 227.3 | 1111.0 |
| (at 19°.5) | (at 110°) | |||
When dealing with gases it is usually more convenient to express the solubility as the ratio of the volume of the gas absorbed to the volume of the absorbing liquid. For gases such as oxygen and nitrogen dissolved in water the solubility as thus defined is independent of the pressure, or the mass of gas dissolved is proportional to the pressure. This relation docs not hold for very soluble gases, such as ammonia, at low temperatures. As a general rule gases are less soluble at high than at low temperatures—unlike the majority of solids. Thus oxygen, 4.89 volumes of which dissolve at atmospheric pressure in 1 volume of water at 0° C, only dissolves to the extent of 3.10 volumes at 20° and 1.70 volumes at 100°.
Cause of Solubility.—At the outset of the subject we are met by a fundamental problem, to which no complete answer can be given: Why do certain substances dissolve in certain other substances and not in different substances? Why are some pairs of liquids miscible in each other in all proportions, while other pairs do not mix at all, or only to a limited extent? No satisfactory correlation of solubility with chemical or other properties has been made. It is possible to state the conditions of solubility in terms of the theory of available energy, but the result comes to little more than a re-statement of the problem in other terms. Nevertheless, such a re-statement is in itself sometimes an advance in knowledge. It is certain then that when dissolution occurs the available energy of the whole system is decreased by the process, while when equilibrium is reached and the solution is saturated the available energy is a minimum. When a variable quantity is at a minimum a slight change in the system does not affect its value, and therefore, when a solution is saturated, the increase in the available energy of the liquid phase produced by dissolving in it some of the solid must be equal to the decrease in the available energy of the solid phase, cam»d by the abstraction from the bulk of that part dissolved. The general theory of such equilibria will be studied later under the head of the phase rule.
It is possible that a correlation may be made between solubility and the energy of surface tension. If a solid is immersed in a liquid a certain part of the energy of the system depends on, and is proportional to, the area of contact between solid and liquid. Similarly with two liquids like oil and water, which do not mix, we have surface energy proportional to the area of contact. Equilibrium requires that the available energy and therefore the area of contact should be a minimum, as is demon- strated in Plateau's beautiful experiment, where a large drop of oil is placed in a liquid of equal density and a perfect sphere is formed. If, however, the energy of surface tension between the two substances were negative the surface would tend to a maximum, and complete mixture would follow. From this point of view the natural solubility of two substances involves a negative energy of surface tension between them.
Gibbs's Phase Rule.—A saturated solution is a system in equili- brium, and exhibits the thermodynamic relations which hold for all such systems. Just as two electrified bodies are in equilibrium when their electric potentials are equal, so two parts of a chemical and physical system are in equilibrium when there is equality between the chemical potentials of each component present in the two parts. Thus water and steam are in equilibrium with each other when the chemical potential of water substance is the same in the liquid as in the vapour. The chemical potentials are clearly functions of the composition of the system, and of its temperature and pressure. It is usual to call each part of the system of uniform composition through- out a phase; in the example given, water substance, the only component is present in two phases—a liquid phase and a vapour phase, and when the potentials of the component are the same in each phase equilibrium exists.
If in unit mass of any phase we have n components instead of one we must know the amount of n−1 components present in that unit mass before we know the exact composition of it. Thus if in one gramme of a mixture of water, alcohol and salt we are told the amount of water and salt, we can tell the amount of alcohol. U, instead of one phase, we have r phases, we must find out the values of r(n−1) quantities before we know the composition of the whole system. Thus, to investigate the composition of the system we must be able to calculate the value of r(n−1) unknown quantities. To these must be added the external variables of temperature and pressure, and then as the total number of variables, we have r (n+1) + 2.
To determine these variables we may form equations between the chemical potentials of the different components—quantities which are functions of the variables to be determined. If μ1 and μ2 denote the potentials of any one component in two phases in contact, when there is equilibrium, we know that μ1=μ2. If a third phase is in equilibrium with the other two we have also μ1=μ3. These two equations involve the third relation μ2=μ3, which therefore is not an independent equation. Hence with three phases we can form two independent equations for each component. With r phases we can form r−1 equations for each component, and with n components and r phases we obtain n(r−1) equations.
Now by elementary algebra we know that if the number of independent equations be equal to the number of unknown quantities all the unknown quantities can be determined, and can possess each one value only. Thus we shall be able to specify the system completely when the number of variables, viz. r(n−1) +2, is equal to the number of equations, viz. r(n−1); that is when r=n+2. Thus, when a system possesses two more phases than the number of its components, all the phases will be in equilibrium with each other at one definite composition, one definite temperature and one definite pressure, and in no other conditions. To take the simplest case of a one component system water substance has its three phases of solid ice, liquid water and gaseous vapour in equilibrium with each other at the freezing point of water under the pressure of its own vapour. If we attempt to change either the temperature or the pressure ice will melt, water will evaporate or vapour condense until one or other of the phases has vanished. We then have in equilibrium two phases only, and the temperature and pressure may change. Thus, if we supply heat to the mixture of ice, water and steam ice will melt and eventually vanish. We then have water and vapour in equilibrium, and, as more heat enters, the temperature rises and the vapour-pressure rises with it. But, if we fix arbitrarily the temperature the pressure of equilibrium can have one value only. Thus by fixing one variable we fix the state of the whole system. This condition is represented in the algebraic theory when we have one more unknown quantity than the number of equations; i.e. when r(n—1) + 2=n(r—1) + 1 or r=n+1, and the number of phases is one more than the number of components. Similarly if we have F more unknowns than we have equations to determine them, we must fix arbitrarily F coordinates before we fix the state of the whole system. The number F is called the number of degrees of freedom of the system, and is measured by the excess of the number of unknowns over the number of variables. Thus F=r(n−1) + 2−n(r−1)=n−r+2, a result which was deduced by J. Willard Gibbs (1839–1903) and is known as Gibbs’s Phase-Rule (see Energetics).
The phenomena of equilibrium can be represented on diagrams. Thus, if we take our co-ordinates to represent pressure and temperature, the state of the systems with Ice, water and vapour in equilibrium is represented by the point O where the pressure is that of the vapour of water at the freezing point and the temperature is the freezing point under that pressure. If all the ice be melted, we pass along the vapour pressure curve of water OA. If all the water be frozen, we have the vapour pressure curve of ice OB; while, if the pressure be raised, so that all the vapour vanishes, we get the curve OC of equilibrium between

Fig. 1.
the pressure and the freezing point of water. The slope of these curves is determined by the so-called "latent heat equation" (see Thermodynamics), dp/dt = λ/t(v2−v1), where p and t denote the pressure and temperature, λ the heat required to change unit mass of the systems from one phase to the other, and v2−v1 the resulting change in volume. The phase rule combined with the latent heat equation contains the whole theory of chemical and physical equilibrium.
Application to Solutions.—In a system containing a solution we have to deal with two components at least. The simplest case is that of water and a salt, such as sodium chloride, which crystallizes without water. To obtain a non-variant system, we must assemble four phases—two more than the number of components. The four phases are (1) crystals of salt, (2) crystals of ice, (3) a saturated solution of the salt in water, and (4) the vapour, which is that practically of water alone, since the salt is non-volatile at the temperature in question. Equilibrium between these phases is obtained at the freezing point of the saturated solution under the pressure of the vapour. At that pressure and temperature the four phases can co-exist, and, as long as all of them are present, the pressure and temperature will remain steady. Thus a mixture of ice, salt and the saturated solution has a constant freezing point, and the composition of the solution is constant and the same as that of the mixed solids which freeze out on the abstraction of heat. This constancy both in freezing point and composition formerly was considered as a characteristic of a pure chemical compound', and hence these mixtures were described as components and given the name of “cryohydrates.”
In representing on a diagram the phenomena of equilibrium in a two-component system we require a third axis along which to plot the composition of a variable phase. It is usual to take three axes at right angles to each other to represent pressure, temperature and the composition of the variable phase. On a plane figure this solid diagram must be drawn in perspective, the third axis C being imagined to lie out of the plane of the paper. The phase-rule diagram that we construct is then a sketch of a solid model, the lines of which do not really lie in the plane of the paper.

Fig. 2.
Let us return to the case of the system of salt and water. At the cryohydric point O we have four phases in equilibrium at a definite pressure, temperature and composition of the liquid phase. The condition of the system is represented by a single point on the diagram. If heat be added to the mixture ice will melt and salt dissolve in the water so formed. If the supply of ice fails first the temperature will rise, and, since solid salt remains, we pass along a curve OA giving the relation between temperature and the vapour pressure of the saturated solution. If, on the other hand, the salt of the cryohydrate fails before the ice the water given by the continued fusion dilutes the solution, and we pass along the curve OB which shows the freezing points of a series of solutions of constantly increasing dilution. If the process be continued till a very large quantity of ice be melted the resulting solution is so dilute that its freezing point B is identical with that of the pure solvent. Again, starting from O, by the abstraction of heat we can remove all the liquid and travel along the curve OD of equilibrium between the two solids (salt and ice) and the vapour. Or, by increasing the pressure, we eliminate the vapour and obtain the curve OF giving the relation between pressure, freezing point and composition when a saturated solution is in contact with ice and salt.
If the salt crystallizes with a certain amount of water as well as with none, we get a second point of equilibrium between four phases. Sodium sulphate, for instance, crystallizes below 32.6° as Na2SO4⋅10H2O, and above that temperature as the anhydrous solid Na2SO4. Taking the point O to denote the state of equilibrium between ice, hydrate, saturated solution and vapour, we pass along OA till a new solid phase, that of Na2SO4, appears at 32.6°; from this point arise four curves, analogous to those diverging from the point O.
For the quantitative study of such systems in detail it is convenient to draw plane diagrams which are theoretically projections of the curves of the solid phase rule diagram on one or other of these planes. Experiments on the relation between temperature and concentration are illustrated by projecting the curve OA of fig. 2 on the tc-plane. The pressure at each point should be that of the vapour, but since the solubility of a solid does not change much with pressure, measurements under the constant atmospheric pressure give a curve practically identical with the theoretical one.

Fig. 3.
Fig. 3 gives the equilibrium between sodium sulphate and water in this way. B is the freezing point of pure water, O that of a saturated solution of Na2SO4⋅10H2O. The curve OP represents the varying solubility of the hydrate as the temperature rises from the cryohydric point to 32.6°. At that temperature crystals of the anhydrous Na2SO4 appear, and a new fixed equilibrium exists between the four phases—hydrate, anhydrous salt, solution and vapour. As heat is supplied, the hydrate is transformed gradually into the anhydrous salt and water. When this process is complete the temperature rises, and we pass along a new curve giving the equilibrium between anhydrous crystals, solution and vapour. In this particular case the solubility decreases with rise of temperature. This behaviour is exceptional.
Two Liquid Components.—The more complete phenomena of mutual solubility are illustrated by the case of phenol and water.

Fig. 4.
In fig. 4 A represents the freezing point of pure water, and AB the freezing point curve showing the depression of the freezing point as phenol is added. At B is a non-variant system made up of ice, solid phenol, saturated solution and vapour. BCD is the solubility curve of phenol in water. At C a new liquid phase appears—the solution of water in liquid phenol, the solubility of which is represented by the curve DE. At D the composition of the two liquids becomes identical, and at temperatures above D, 68°C the liquids are soluble in each other in all proportions, and only one liquid phase can exist. If the two substances are soluble in each other in all proportions at all temperatures above their melting points we get a diagram reduced to the two fusion curves cutting each other at a non-variant point. This behaviour is illustrated by the case of silver and copper (fig. 5). At the non-variant point the two metals freeze out together and the composition of the liquid is the same as that of the mixed solid which crystallizes from it. The solid is then known as a eutectic alloy.
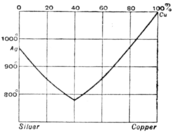
Fig. 5.
A liquid in which the composition is nearly that of the eutectic shows the changes in the rate of fall of temperature as it is allowed to cool. First a small quantity of one of the pure components begins to crystallize out, and the rate of cooling is thereby diminished owing to the latent heat liberated by the change of state. This process continues till the composition of the liquid phase reaches that of the eutectic, when the whole mass solidifies on the further loss of heat without change of temperature, giving a very definite freezing point. The process of cooling is thus represented by a path which runs vertically downwards till it cuts the freezing point curve, and then travels along it till the non-variant point is reached. In this way two temperature points are obtained in the investigation—the higher giving a point on the equilibrium curve, the lower showing the non-variant point.
Other pairs of alloys, showing more complicated relations, are described in Alloy. Experiments on alloys are, in some ways, easier to make than on pairs of non-metallic substances, partly owing to the possibility of polishing sections for microscopic examination, and the investigation of alloys has done much to elucidate the general phenomena of solution, of which metallic solution constitutes a special case.
When the two components form chemical compounds with each other, the phenomena of mutual solubility become more complex.

Fig. 6.
For a simple case to serve as an introduction, let us again turn to alloys. Copper and antimony form a single compound SbCu2. If either copper or antimony be added to this compound, the freezing point is lowered just as it would be if a new substance were added, to a solvent. Thus on each side of the point B representing this compound, the curve falls. Proceeding along the curve in either direction, we come to a non-variant or eutectic point. In one case (represented by the point A in the figure) the solid which freezes out is a conglomerate of crystals of the compound with those of antimony, in the other case C with those of copper. Thus in interpreting complicated freezing point curves, we must look for chemical compounds where the curve shows a maximum, and for a eutectic or cryohydrate where two curves meet at a minimum point.
We are now ready to study a case where several compounds are formed between the two components. A good example is the

Fig. 7.
equilibrium of ferric chloride and water, studied by B. Roozeboom. The experimental curve of solubility is shown in fig. 7. At A we have the freezing point of pure water, which is lowered by the gradual addition of ferric chloride in the manner shown by the curve AB. At B we have the non-variant cryohydric point at which ice, the hydrate Fe2 Cl c -12H 2 0, the saturated solution and the vapour are in equilibrium at 55 C. As the proportion of salt is increased, the melting point of the conglomerate rises, till, at the maximum point C, we have the pure compound the hydrate with twelve molecules 30 of water. Beyond C, the Fig. 7. addition of salt lowers the melting point again, till at D we obtain another non-variant point. This indicates the appearance of a new compound, which should exist pure at E, the next maximum, and, led by these considerations, Roozeboom discovered and isolated a previously unknown hydrate, Fe 2 Cl 6 7-H 2 0. In a similar way the curve FGH, between 30 and 55, shows the effect of the hydrate Fe 2 Cl 6 -5H 2 0, and the curve HJK that of the hydrate Fe 2 Cl 6 -4H 2 0, which, when pure, melts at 73·5°—the point J on the diagram. At the point K, 66°, begins the solubility curve of the anhydrous salt, Fe 2 Cl6, the fusion point of which when pure is beyond the limits of the diagram. Let us now trace the behaviour of a solution of ferric chloride which is evaporated to dryness at a constant temperature of 31°. The phenomena may be investigated by following a horizontal line across the diagram. When the curve BC is reached, Fe 2 C16-12H 2 separates out, and the solution solidifies. Further renewal of water will cause first liquefaction, as the curve CD is passed, and then resolidification to Fe 2 Cl 6 -7H 2 when DE is cut. Again the solid will liquefy and once more become solid as Fe 2 CU-5H 2 0. Still further evaporation causes these crystals to effloresce and pass into the anhydrous salt. As we have seen, the maxima of the various curve-branches at C, E, G, and J correspond with the melting points of the various hydrates at 37º, 32·5°, 56° and 73·5° respectively; and at these points melting or solidification of the whole mass can occur at constant temperature. But we have also found this behaviour to be characteristic of the nonvariant or transition points, which, in this case, are represented by the points B,D,F,Hand K (-55º, 27·4°, 30°, 55° and 66°). Thus in two ways at least a constant melting point can be obtained in a two-component system.
Solid Solutions.—In all the cases hitherto considered, the liquid phase alone has been capable of continuous variation in composition. The solid phases each have been of one definite substance. Crystals of ice may lie side by side with crystals of common salt, but each crystalline individual is either ice or salt; no one crystal contains both components in proportions which can be varied continuously. But, in other cases, crystals are known in which both components may enter. Such phenomena are well known in the alums—double sulphates of aluminium with another metal. Here the other metal may be one, such as potassium, or two, such as potassium and sodium, and, in the latter case, the proportion between the two may vary continuously throughout wide limits. Such structures are known as mixed crystals or solid solutions.
The theoretical form of the freezing point diagrams when solid solutions are present depends on the relation between the available energy and the composition in the two phases. This relation is known when the amount of either component present in the other is very small, for it is then the relation for a dilute system and can
 |
 |
 |

|
| Fig. 8. | Fig. 9. | Fig. 10. | Fig. 11. |
be calculated. But at intermediate compositions we can only guess at the form of the energy-composition curve, and the freezing point composition curve, deduced from it, will vary according to the supposition which we make. With the most likely forms for the energy curves we get the accompanying diagrams for the relation between freezing point and concentration.
It will be noticed that in all these theoretical curves the points of initial fusion and solidification do not in general coincide; we reach a different curve first according as we approach the diagram from below, where all is solid, or from above, where all is liquid. Again, it will be seen that the addition of a small quantity of one component, say B, to the other, A, does not necessarily lower the melting point, as it does with systems with no solid solutions; it is quite as likely to cause it to rise. The second and third figures, too, show that the presence of solid solutions may simulate the phenomena of chemical combination, where the curve reaches a maximum, and of non-variant systems where we get a minimum. The fourth figure shows that, in some cases, it should be possible for solid solutions to be present in a limited part of the field only, being absent between the two nearly vertical lines in fig. 11. Experiment has revealed the existence of systems in which these phenomena are displayed. As an example we may take the case of mixtures of naphthalene and /3-naphthol, substances which form solid solutions in each other. The freezing and melting point curves are exactly similar to theoretical curves of fig. 8, the point A representing pure naphthalene and B pure /3-naphthol. When the equilibria become more complex difficulties of interpretation of the experimental results often arise. It is often very difficult to distinguish between a chemical compound, for example, and the case of solid solution represented by fig. 9. All available evidence, from the freezing point curve and from other sources must be scrutinized before an opinion is pronounced. But the elucidation of the complicated phenomena of solid solutions would have been impossible without the theoretical knowledge deduced from the principle of available energy.
Supersaturation. â€" When a crystal of the solid phase is present the equilibrium of a solution is given by the solubility curves we have studied. If, however, a solution be cooled slowly past its saturation point with no solid present, crystallization does not occur till some lower temperature is reached. Between the saturation point and this lower temperature, the liquid holds in solution more of the solute than corresponds with equilibrium, and is said to be supersaturated. A familiar example is to be found in solutions of sodium sulphate, which may be cooled much below their saturation point and kept in the liquid state till a crystal of the hydrate NaaS(VioH 2 is dropped in, when solidification occurs with a large evolution of latent heat. These phenomena are explicable if we consider the energy relations,
for the intrinsic energy of a system will contain terms depending on the area of contact between different phases, and, for a given mass of material, the area will be greater if the substance is finely divided. Hence the conditions necessary to secure equilibrium when the solid phase is present are not the same as those necessary to cause crystallization to start in a number of crystals at first excessively minute in size. The corresponding phenomenon in the case of vapours is well known. Dust-free air will remain supersaturated with water-vapour in conditions where a dense cloud would be formed in presence of solid dust- nuclei or electric ions which serve the same purpose.
If a solution of a salt be stirred as it cools in an open vessel, a thin shower of crystals appears at or about the saturation temperature. These crystals grow steadily, but do not increase in number. When the temperature has fallen about io° C. below this point of saturation, a dense shower of new crystals appear suddenly. This shower may be dense enough to make the liquid quite opaque. These phenomena have been studied by H. A. Miers and Miss F. Isaac. If the solution be confined in a sealed glass tube, the first thin shower is not formed, and the system remains liquid till the secondary dense shower comes down. From this and other evidence it has been shown that the first thin shower in open vessels is produced by the accidental presence of tiny crystals obtained from the dust of the air, while the second dense shower marks the point of spon- taneous crystallization, where the decrease in total available energy caused by solidification becomes greater than the increase due to the large surface of contact between the liquid and the potentially existing multitudinous small crystals of the shower.
If the temperature at which this dense spontaneous shower of crystals is found be determined for different concentrations of solution, we can plot a " supersolubility curve," which is found generally to run roughly parallel to the " solubility curve " of steady equilibrium between liquid and already existing solid. When two substances are soluble in each other in all proportions, we get solubility curves like those of copper and silver shown in fig. 5. We should expect to find supersolubility curves lying below the solubility curves, and this result has been realized experimentally for the supersolubility curves of mixtures of salol (phenyl salicylate) and betol (/3-naphthol salicylate) represented by the dotted lines of fig. 12.
In practical cases of crystallization in nature, it is probable that these phenomena of supersaturation often occur. If a liquid mixture
of A and B (fig. 12) were inocu- lated with crystals of A when its composition was that represented by x, cooled very slowly and stirred, the conditions would be those of equilibrium throughout. When the temperature sank to a, on the freezing point curve, crystals of pure A would appear. The residual liquid would thus become richer in B, and the tem- perature and composition would pass along the curve till E, the eutectic point, was reached. The 100 liquid then becomes saturated " with B also, and, if inoculated with B crystals, will deposit B alongside of A, till the whole mass is solid. But, if no solid be present initially, or if the cooling be rapid, the liquid of composition x becomes supersaturated and may cool till the supersaturation curve is reached at b, and a cloud of A crystals comes down. The temperature may then rise and the concentration of B increase in the liquid in a manner represented by some such line as b f. The conditions may then remain those of equilibrium along the curve / E, but before reaching / the solution may become supersaturated with B and deposit B crystals spontaneously. The eutectic point may never be reached. The possibility of these phenomena should be borne in mind when attempts are made to interpret the structure of crystalline bodies in terms of the theory of equilibrium.
Osmotic Pressure. — The phase rule combined with the latent heat equation enables us to trace the general phenomena of equilibrium in solutions, and to elucidate and classify cases even of great complexity. But other relations between the different properties of solutions have been investigated by another series of conceptions which we shall proceed to develop. Some botanical experiments made about 1870 suggested the idea of semi-permeable membranes, i.e. membranes which allow a solvent to pass freely but are impervious to a solute when dissolved in that solvent. It was found, for instance, that a film of insoluble copper ferrocyanide, deposited in the walls of a
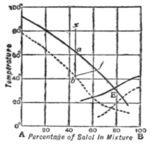
Fig. 12.
porous vessel by the inward diffusion and meeting of solutions of copper sulphate and potassium ferrocyanide, would allow water to pass, but retained sugar dissolved in that liquid. It was found, too, when water was placed on one side of such a membrane, and a sugar solution in a confined space on the other, that water entered the solution till a certain pressure was set up when equilibrium resulted.
The importance of these experiments from the point of view of the theory of solution, lay in the fact that they suggested the con- ception of a perfect or ideal semi-permeable partition, and that of an equilibrium pressure representing the excess of hydrostatic pressure required to keep a solution in equilibrium with its pure solvent through such a partition. Artificial membranes are seldom or never perfectly semi-permeable — some leakage of solute nearly always occurs, but the imperfections of actual membranes need no more prevent our use of the ideal conception than the faults of real engines invalidate the theory of ideal thermodynamics founded on the conception of a perfect, reversible, frictionless, heat engine. Further, in the free surface the solutions of an involatile solute in a volatile solvent, through which surface the vapour of the solvent alone can pass, and in the boundary of a crystal of pure ice in a solution, we have actual surfaces which are in effect perfectly semi- permeable. Thus the results of our investigations based on ideal conceptions are applicable to the real phenomena of evaporation and freezing.
Dilute Solutions. — Before considering the more complicated case of a concentrated solution, we will deal with one which is very dilute, when the theoretical relations are much simplified. The vapour pressure of a solution may be p^ssare. measured experimentally by two methods. It may be compared directly with that of the pure solvent, as the vapour- pressure of a pure liquid is determined, by placing solvent and solution respectively above the mercury in two barometer tubes, and comparing the depressions of the mercury with the height of a dry barometer at the same temperature. This method was used by Raoult. On the other hand, a current of dry air may be passed through the series of weighed bulbs containing solution and solvent respectively, and the loss in weight of each determined. The loss in the solution bulbs gives the mass of solvent absorbed from the solution, and the loss in the solvent bulbs the additional mass required to raise the vapour pressure in the air-current to equilibrium with the pure solvent. The relative lowering of vapour pressure of the solution compared with that of the solvent is measured by the ratio of the extra mass absorbed from the solvent bulbs to the total mass absorbed from both series of bulbs. Experiments by this method have been made by W. Ostwald and J. Walker, and by Lord Berkeley and E. G. J. Hartley.
The vapour pressure of the solution of a non- volatile solute is less than the vapour pressure of the pure solvent. Hence if two vessels, one filled with solvent and one with solution, be placed side by side in an exhausted chamber, vapour will evapo- rate from the solvent and condense on the solution. The solution will thus gain solvent,, and will grow more and more dilute. Its volume will also increase, and thus its upper surface will rise in the vessel. But as we ascend in an atmosphere the pressure diminishes; hence the pressure of the vapour in the chamber is less the higher we go, and thus eventually w T e reach a state of equilibrium where the column of vapour is in equilibrium at the appropriate level both with solvent and solution. Neglecting the very small buoyancy of the vapour, the hydrostatic pressure P at the foot of the column of solution is h g p where h is the height of the column and p the mean density of the solution. If the height be not too great, we may assume the density of the vapour to be uniform, and write the difference in vapour pressure at the surfaces of the solvent and of the solution as p — p'= hgcr. Hence we find that p — p' =P<r/p for a very dilute solution, where the difference p—p' is small and the height of the balancing column of solution small.
In practice the time required to reach these various conditions of equilibrium would be too great for experimental demonstration, but the theoretical consideration of vapour pressures is of funda- mental importance. Let us suppose that we possess a partition such as that described above, which is permeable to the solvent but not to the solute when dissolved in it, and let us connect the solution and solvent of fig. 13 with each other through such a partition. If solvent were to flow one way or the other through the partition, the height of the column of solution would rise or fall and the equilibrium with the vapour be disturbed. A continual circulation might thus be set up in an isothermal enclosure and maintained with the performance of an unlimited supply of work. This result would be contrary to all experience of the impossibility of "perpetual motion," and hence we may conclude that through such a semi-permeable wall, the solvent and the solution at the foot of the column would

Fig. 13.
be in equilibrium under the excess of hydrostatic pressure represented when the solution is very dilute by P = (p-p1)p/<r. But such a pressure represents the equilibrium osmotic pressure discussed above. Therefore the equilibrium osmotic pressure of a solution is connected with the vapour pressure, and, in a very dilute solution, is expressed by the simple relation just given.
Another relation becomes evident if we use as a semi-permeable partition a "vapour sieve" as suggested by G. F. Fitzgerald. If a number of small enough holes be drilled through a solid substance which is not wetted by the liquid, our knowledge of the phenomena of capillarity shows us that it needs pressure to force the liquid into the holes. A piston made of such a perforated substance, therefore, may be used to exert pressure on the liquid, while all the time the vapour is able to pass. By evaporation and condensation, then, the solvent can pass through this perforated partition, which thus acts as a perfect semi-permeable membrane. When the solution and solvent are in equilibrium across the partition, the vapour pressure of the solution has been increased by the application of pressure till it is equal to that of the solvent. In any solution, then, the osmotic pressure represents the excess of hydrostatic pressure which it is necessary to apply to the solution in order to increase its vapour pressure to an equality with that of the solvent in the given conditions.
Similar considerations show that, since at its freezing point the vapour pressure of a solution must be in equilibrium with that of ice, the depression of freezing point produced by dissolving a sub- stance in water can be calculated from a knowledge of the vapour pressure of ice and water below the freezing point of pure water. But another method of investigation will illustrate new ways of treating our subject.
By imagining that a dilute solution is put through a thermo- dynamic cycle we may deduce directly relations between its osmotic pressure and its freezing point. Let us Point, freeze out unit mass of solvent from a solution at its freezing point T— dT and remove the ice, which is assumed to be the ice of the pure solvent. Then let us heat both ice and solution through the infinitesimal temperature range dT to the freezing point T of the solvent, melt the ice by the application of an amount of heat L, which measures its latent heat of fusion, and allow the solvent so formed to enter the solution reversibly through a semi-permeable wall into an engine cylinder, doing an amount of work Vdv. By cooling the resultant solution through the range dT we recover the original state of the system. The well-known expression for the efficiency of the cycle of reversible operation gives us
PdvfL = dT/T or dT = TPdv/L
as a value for the depression of the freezing point of the solution compared with that of the pure solvent.
The freezing point of a solution may be determined experimentally. The solution is contained in an inner tube, surrounding which is an air space. Then comes an outer vessel, in which a freezing mixture can be placed. This solution is stirred continuously and the tem- perature falls slowly below the freezing point, till the supersaturation point is reached, or until a crystal of ice is introduced. The solution then freezes, until the heat liberated is enough to raise the temperature to the point of equilibrium given by the tendency of the solution taken in contact with ice to approach the true freezing point on one side and the temperature of the enclosure on the other. To get the true freezing point then, it is well to arrange that the temperature of the enclosure should finally be nearly that of the freezing point to be observed. One way in which this has been secured is by obtaining the under cooling by temporary cooling of» the air space by a spiral tube in which ether may be evaporated, the outer vessel being filled with ice in contact with a solution of equivalent concentration to that within. Modifications of this method have been used by many observers, among others by Raoult, Loomis, H. C. Jones, and by_ E. H. Griffiths and T. G. Bedford, who compared directly the freezing points of dilute solutions with those of the pure solvent in similar conditions by the accurate methods of platinum thermometry.
Another application of the theory of energy enables us to co- ordinate the osmotic pressure of a dilute solution with the pressure of a gas occupying the same space. On Absolute the fundamental hypotheses of the molecular theory, Value ot we must regard a solution as composed of a number Osmotic of separate particles of solute, scattered through- out the solvent. Each particle may react in some way on the solvent in its neighbourhood, but if the solution be so dilute that each of these spheres of influence is unaffected by the rest, no further addition of solvent will change the connexion between one particle of solute and its associated solvent. The only effect of adding solvent will be to separate further from each other the systems composed of solute particle as nucleus and solvent as atmosphere; it will not affect the action of each nucleus on its atmosphere. Thus the result will be the same whatever the nature of the inter- action may be. If solvent be allowed to enter through a semi- permeable wall into an engine cylinder, the work done when the solution within is already dilute will be the same whatever the nature of the interaction between solute and solvent, that is, whatever be the nature of the solvent itself. It will even be the same in those cases where, with a volatile solute, the presence of a solvent may be dispensed with, and the solute exist in the same volume as a gas. Now the work done by allowing a small quantity of solvent to enter reversibly into an osmotic cylinder is measured by the product of the osmotic pressure into the change in volume. Hence the osmotic pressure is measured by the work done per unit change of volume of the solution. The result of our consideration, therefore, is that the osmotic pressure of a dilute solution of a volatile solute must have the same value as the gaseous pressure the same number of solute particles would exert if they occupied as gas a volume equal to that of the solution.
The reasoning given above is independent of the temperature, so that the variation with temperature of the osmotic pressure of a dilute solution must be the. same as that of a gas, while Boyle's law must equally apply to both systems. Experimental evidence confirms these results, and extends them to the cases of non-volatile solutes — as is, indeed, to be expected, since volatility is merely a matter of degree. When the solution ceases to be dilute in the thermodynamic sense of the word, that is, when the spheres of influence of the solute particles intersect each other, this reasoning ceases to apply, and the resulting modifica- tion of the gas laws as applied to solutions becomes a matter for further investigation, theoretical or experimental. In the limit then, when the concentration of the solution becomes vanishingly small, theory shows that the osmotic pressure is equal to the pressure of a gas filling the same space. Experiments with membranes of copper ferrocyanide have verified this result for solutions of cane-sugar of moderate dilutions. But the most accurate test of the theory depends on measurements of freezing points.
A quantity of gas measured by its molecular weight in grammes when confined in a volume of one litre exerts a pressure of 22-2 atmospheres, and thus the osmotic pressure of a dilute solution divided by its concentration in gramme-molecules per litre has a corresponding value. But we have seen that the depression of dT of the freezing poinjt of a dilute solution is measured by TPdv/h. Putting the absolute temperature of the freezing point of water as 273 °, the osmotic pressure P as 22-2 atmospheres or 22-4Xlo 6 , C.G.S. units per unit concentration, L the latent heat as 79-4 X 4-I84XI0 7 in the corresponding units, and dv the volume change in the solution for unit mass of solvent added we get for the quantity d l jc, where c is the concentration of the solution, the value i-857° C. per unit concentration. Experimental measurements of freezing points of various non-electrolytic solutions have been made by Raoult, Loomis, Griffiths, Bedford and others and numbers ranging round 1-85 found for this concentration. Equally good comparisons have been obtained for solutions in other solvents such as acetic acid 3-88, formic acid 2-84, benzene 5-30, and nitrobenzene 695. Such a concordance between theory and experiment not only verifies the accuracy of thermodynamic reasoning as applied to dilute solutions, but gives perhaps one of the most convincing experimental verifications of the general validity of thermodynamic theory which we possess.
Another verification may be obtained from the phenomena of vapour pressure. Since, in dilute solutions, the osmotic pressure has the gas value, we may apply the gas equation PV = nRT = npvi to osmotic relations. Here n is the number of gramme-molecules of solute, T the absolute temperature, R the gas constant with its usual " gas " value, p the vapour pressure of the solvent and Hi the volume in which one gramme-molecule of the vapour is confined. In the vapour pressure equation p~p' = P<r//>, we have the vapour density a equal to M/Vi, where M is the molecular weight of the solvent. The density of the liquid is MN/V, where N is the number of solvent molecules, and V the total volume of the liquid. Substituting these values, we find that the relative lowering of vapour pressure in a very dilute solution is equal to the ratio of the numbers of solute and solvent molecules, or {p â€" p')/p = »/N.
The experiments of Raoult on solutions of organic bodies in water and on solutions of many substances in some dozen organic solvents have confirmed this result, and therefore the theoretical value of the osmotic pressure from which it was deduced.
Although even good membranes of copper ferrocyanide are rarely perfectly semi-permeable, and in other membranes such as indiarubber, &c, which have been used, the defects from the theoretical values of the equilibrium pressure are very great, yet, in the light of the exact verification of theory given by the experiments described above, it is evident that such failures to reach the limiting value in no wise invalidate the theory of osmotic equilibrium. They merely show that, in the conditions of the particular experiments, the thermodynamic equilibrium value of the osmotic pressure cannot be reached â€" the thermodynamic or theoretical osmotic pressure (which must be independent of the nature of the membrane provided it is truly semi-permeable) is a different thing from the equilibrium pressure actually reached in a given experiment, which measures the balance of ingress and egress of solvent through an imperfect semi-permeable membrane.
Dilute solutions of substances such as cane-sugar, as we have seen, give experimental values for the connected osmotic properties â€" pressure, freezing point and vapour a^trofytes. pressure â€" in conformity with the theoretical values. All these solutions are non-conductors of electricity. On the other hand, solution of mineral acids and salts conduct the current with chemical decompositionâ€" they are called electrolytes. In order to explain the electrical properties of a solution, for instance of potassium chloride, we are driven to believe that each molecule of the salt is dissociated into two parts, potassium and chlorine, each associated with an electric charge equal in amount but opposite in sign. The movement in opposite directions of these charged ions constitutes the electric current in the solution. To explain the electrical properties of sulphuric acid in aqueous solution, the supposition of three ions, two of hydrogen and one of the chemical group SO4, is necessary. Now measurements of osmotic properties of these solutions show that their osmotic pressures are abnormally great and that, at extreme dilution, the ratio of their osmotic pressures to that of equivalent solutions of non-electrolytes is equal to the number of ions indicated by the electrolytic properties. From the osmotic side also, then, electrolytic dissociation is indicated, and indeed, it was from this side that the idea was first suggested by S. Arrhenius in 1887. The subject is dealt with in Electrolysis and Conduction, Electric: § In Liquids.
Concentrated Solutions. â€" Having dealt with the relations between the properties of an ideally dilute solution, we now turn to the consideration of the general case where the simplifying assumption of great dilution is not made.
The height of the column of solution in -fig. 13 required for osmotic equilibrium through a semi-permeable wall below is now very great, since the osmotic pressure of strong solutions may reach many hundred atmospheres. Hence we must not assume that the density of the vapour in the surrounding atmosphere is constant, or that the solution, when equilibrium is reached, is of uniform concentration throughout. The osmotic pressure (defined as the difference in the hydrostatic pressures of the solution and solvent when pressure. their vapour pressures are equal and they are consequently in equilibrium through a perfect semi-permeable membrane) may also depend on the absolute values of the hydrostatic pressures, as may the vapour pressure of the liquids.
To investigate the osmotic pressure of a strong solution we may consider the hydrostatic pressure required to increase its vapour pressure to an equality with that of the solvent. The relation between hydrostatic pressure and the vapour pressure of a pure liquid may be obtained at once by considering the rise of liquid in a capillary tube. The difference in vapour pressure at the top and at the bottom of the column is pâ€"p' =Pa/p, as shown above for a column of solution. Writing v for 1/0-, the specific volume of the vapour at the pressure p, and V for i/p, the specific volume of the liquid at the pressure P, and restricting the result to small changes, we get vdp^VdP.
In considering the corresponding relation for a solution instead of a pure liquid, possible differences in concentration make the column method difficult of application, and it is better to attach the problem by means of an imaginary cycle of isothermal operation. The simplest way to do this is to imagine a vapour-sieve piston through which the vapour but not the liquid can pass. As we have explained above, such a vapour sieve may be constructed by boring a number of small enough holes through a solid not wetted by the liquid.
Let us imagine unit mass of solution of volume V confined in a cylinder ABC between a fixed vapour sieve B and a solid piston A

Fig. 14.
by which a pressure P is applied. The vapour at pressure p in equilibrium with the liquid is bounded by a solid piston C, which we can also move to change the pressure or volume.
With such an imaginary apparatus, H. L. Callendar has shown that the variation of vapour pressure of a solution with pressure is given by the expression WdP=vdp, where V is the change in volume of the solution when unit mass of solvent is mixed with it. The corresponding relation for a pure liquid can be regained by considering that at infinite dilution the liquid becomes pure solvent, and the change of volume becomes equal to the volume V of solvent added.
The osmotic pressure Po is the difference of the hydrostatic pressures P' and P of the solution and the solvent when their vapour pressures are equal. Hence <2P = iP'-dP and dP /iP = (V-V')/V' or dPo/dP' = (Vâ€" V')/V. If V = V there is no change in osmotic pressure with hydrostatic pressure, and osmotic pressure depends on concentration and temperature only.
The relation between the equilibrium pressures P and P' for solution and solvent corresponding to the same value po of the vapour pressure is obtained by integrating the equation V'dP' = vdp between corresponding limits for solution and solvent. We get
fP'W'dP' = fP° vdp and /"P VJP = fPc vdp,
J P' J Pi J P J P
whence / *', ™ P ' " f] VdP = /* «**
where p and p' are the vapour pressures of solvent and solution each under its own vapour pressure only.
If we measure the osmotic pressure Po when the solvent is under its own vapour pressure only, that is, when P = p = po, the term involving V vanishes, and the limit of integration P' becomes Po+£. If we assume that V, the volume change on dilution, varies regularly or not appreciably with pressure, we may write the first integral as V(JPo+pâ€"p') where V' now denotes its mean value between the limits.
To evaluate the second integrals vdp we may subtract a constant b to represent the defect of the volume of the vapour from the ideal volume R//£. This gives
V'{Vo+p-p') = Rf log (pip') -b(p-p').
For most experimental purposes the small terms involving the
factor {pâ€"p') may be neglected, and we have, approximately,
PoV' = R(log (pip').
From this equation the osmotic pressure Po required to keep a solution in equilibrium as regards its vapour and through a semi-permeable membrane with its solvent, when that solvent is under its own vapour pressure, may be calculated from the results of observations on vapour pressure of solvent and solution at ordinary low hydrostatic pressures. The chief difficulty lies in the determination of the quantity V, the change in volume of the solution under the pressure Po when unit mass of solvent is mixed with it. This determination involves a knowledge of the density and of the compressibility of the solution; the latter property is difficult to measure accurately.
In some solutions such as those of sugar the change in volume on dilution is nearly equal to the volume of solvent added; V then becomes equal to V, the specific volume of the solvent. The osmotic pressures of strong sugar solutions were measured successfully by a direct method with semi-permeable membranes of copper ferrocyanide by Lord Berkeley and E. G. J. Hartley, who also determined the vapour pressures by passing a current of air successively through weighed vessels containing solution and water respectively.
Their table of comparison published in 1906 shows the following agreement: â€"
A table should appear at this position in the text. See Help:Table for formatting instructions. |
Concentration in
grammes per litre of
solution.
Osmotic pressure at o° C. in atmospheres.
From vapour pressures.
From direct measurement.
420 540 660 750
44-3 (at 12-6°)
69-4 101-9 136-0
43-97
67-5I
100-78
133-74
It seems likely that measurements of vapour pressure and compressibility may eventually enable us to determine accurately osmotic pressures in cases where direct measurement is impossible.
The slope of the temperature vapour pressure curves in the neighbourhood of the freezing point of the solvent is given by Freezing the latest heat equation. The difference in the two Pointâ€" slopes for water and ice is dp/dT â€" dp' fd T = L/Tti, Solutions. wne re l i the latent heat of fusion, is the difference between the heats of evaporation for ice and water, and v is the specific volume of the vapour.
The difference in the lowering of vapour pressures dpâ€" dp' may be put equal to V<ZP/z>, where P is the osmotic pressure, and V the specific volume of the solvent. We then get VrfP = LdT/T.
In order to integrate this expression we need to know L and v as functions of the temperature and pressure. The latent heat L
at any temperature is given by L = Loâ€" \ g (s-s')dT, where Lo
is value at T and'sâ€"s' is the difference in the specific heats of water and ice. The probable error in neglecting any variation of specific heat is small, and we may calculate L from the values of Lo â€" (s~- s') (T â€" T), where'sâ€"s' is about 0-5 calories. The variation of L with pressure is probably small.
The volume of a gramme of water also depends on temperature and pressure. Approximately one degree lowering of freezing point corresponds with a change of 12 atmospheres in the osmotic pressure. From the known coefficients of compressibility and thermal expansion we find that V may be represented by the linear equation V = 1-000+0-0008 A, where A is the lowering of the freezing point below 0°.
Putting in these values and integrating we have, neglecting terms involving A\ P = 12-o6 Aâ€" 0-021 A* where P is the osmotic pressure in atmospheres.
H. W. Morse and J. C. W. Frazer, who have made direct measurements of osmotic pressure of solution of cane-sugar, have also measured the freezing points of corresponding solutions. From these results the equation just given has been examined by G. N. Lewis.
A table should appear at this position in the text. See Help:Table for formatting instructions. |
Concentration in gramme- molecules per litre of water.
Depression of the freezing point = A.
Osmotic pressure.
Calculated from A.
Observed.
01 o-5
I-O
0-195
0-985 2-07
2-35 n-8 249
2-44 n-8 24-8
Thus the theory of the connexion of osmotic pressure with freezing point (like that with vapour pressure) seems to give results which accord with experiments.
At the limit of dilution, when the concentration of a solution approaches zero, we have seen that thermodynamical theory, verified by experiment, shows that the osmotic frvssure. P ressure nas the same value as the gas pressure of the same number of molecules in the same space. Gases at high pressures fail to conform to Boyle's law, and solutions at moderate concentrations give osmotic pressures which increase faster than the concentration. The variation of gases from Boyle's law is represented in the equation of Van der Waals by subtracting a constant b from the total volume to represent the effect of the volume of the molecules themselves. The corresponding correction in solutions consists in counting only the volume of the solvent in which the solute is dissolved, instead of the whole volume of the solution.

Fig. 15.
In fig. 15 the curve I represents Boyle's law if the volume is taken to be that of the solution, and the curve II if the volume is that of the solvent. Even this correction is not sufficient in solution of sugar, where the theoretical curve II lies below the experimental observations. A further correction may be made by adding more empirical terms to the equation, but a more promising idea, due to J. H. Poynting and H. L. Callendar is to trace the effect of possible combination of molecules of solute with molecules of the solvent. These combined solvent molecules are thus removed from existence as solvent, the effective volume of which is reduced to that of the remaining free molecules of solvent. The greater the number of water molecules attached to one sugar molecule, the less the residual volume, and the greater the theoretical pressure. Callendar finds that five molecules of water in the case of cane-sugar or two molecules in the case of dextrose are required to bring the curves into conformity with the observations of Berkeley and Hartley, which in fig. 15 are indicated by crosses.
Solubility and Heat of Solution. â€" The conceptions of osmotic pressure and ideal semi-permeable membranes enable us to deduce other thermodynamic relations between the different properties of solutions. As an example, let us take the following investigation: â€"
An engine cylinder may be imagined to possess a semi-permeable bottom and to work without friction. If it be filled with a solution and the bottom immersed in the pure solvent, pressure equal to the osmotic pressure must be exerted on the piston to maintain equilibrium. Such a system is in the thermodynamic equilibrium. The slightest change in the load will cause motion in one direction or the other â€" the system is thermodynamically reversible. Such an arrangement may be put through a cycle of operations as in Carnot's engine (see Thermodynamics) and all the laws of reversible engines applied to it. If the solution in the cylinder be kept saturated by the presence

Fig. 16.
of crystals of the solute, crystals will dissolve as solvent enters, and the solution remains saturated throughout. By an imaginary cycle of operations we may then justify the application to solutions of the latent heat have already assumed as applicable. \/T(v2â€"i>i), P is the osmotic pressure, ture and X the heat of solution of when dissolving to form a volume equation which we In the equation dP/dT = T the absolute temperaunit mass of the solute of saturated solution in an osmotic cylinder. This process involves the performance of
an amount of osmotic work P(»2 — Vi). If the heat of solution be measured in a calorimeter, no work is done, so that, if we call this calorimetric heat of solution L, the two quantities are connected by the relation L = X + P(f2— Vi). If L is zero or negligible, X=^P(» 2 -!; 1 ) and we have <2P/<2T=-P/T or dP/P=-dT/T, which on integration gives log P = log T + C, or P = &T, i.e. the osmotic pressure is proportional to the absolute temperature. This result must hold good for any solution, but if the solution be dilute when saturated, that is, if the solubility be small, the equation shows that if there be no heat effect when solid dissolves to form a saturated solution, the solubility is independent of temperature, for, in accordance with the gas laws, the osmotic pressure of a dilute solution of constant concentration is proportional to the absolute temperature. It follows that if the thermodynamic heat of solution be positive, that is, if heat be absorbed to keep the system at constant temperature, the solubility will increase with rising temperature, while if heat be evolved on dissolution, the solubility falls when the system is heated.
In all this investigation it should be noted that the heat of solu- tion with which we are concerned is the heat effect when solid dissolves to form a saturated solution. It is not the heat effect when solid is dissolved in a large excess of solvent, and may differ so much from that effect as to have an opposite sign. Thus cupric chloride dissolves in much water with an evolution of heat, but when the solution is nearly saturated, it is cooled by taking up more of the solid.
In a very dilute solution no appreciable heat is evolved or absorbed when solvent is added, but such heat effects are Osmotic generally found with more concentrated solutions. Pressure The result is to change the relation between tempera- and Tem- ture and the osmotic pressure of a solution of constant pera ure. concen tration, a relation which, in very dilute solutions, is a direct proportionality.
The equation of available energy (see Energetics) A = U + TdA/dT may be applied to this problem. The available energy A is the work which may be gained from the system by a small rever- sible isothermal operation with an osmotic cylinder, that is Pdv. If I is the heat of dilution per unit change of volume in a calorimeter where all the energy goes to heat, the change in internal energy U is measured by Idv. We then have
Pdv=ldv+T-^{Pdv).
Neglecting the volume change with temperature this gives P =l-\-TdP/dT for the relation required. In the case where / is negligible we have P/dP = T/dT, which on integration shows that the osmotic pressure, as in the special case of a dilute solution, is proportional to the absolute temperature.
Theories of Solution. — The older observers, noticing the heat effects which often accompany dissolution, regarded solutions as chemical compounds of varying composition. The physical investigation of osmotic pressure, and its correlation by Van't Hoff with the pressure of a gas, brought forward a new aspect of the phenomena, and suggested an identity of physical modus operandi as well as of numerical value. On this view, the function of the solvent is to give space for the solute to diffuse, and the pressure on a semi-permeable membrane is due to the excess of solvent molecules entering over those leaving in conse- quence of the smaller number which impinge on the membrane from the side of the solution; the defect in the number must be proportional, roughly at any rate, to the number of solute molecules, present, that is, to the strength of the solution.
Whatever view, if any, be adopted as to the nature of a solu- tion, the thermodynamic relations we have investigated equally hold good. It is the strength and weakness of thermodynamic methods that they are independent of theories of constitution. The results are true whatever theory be in vogue, but the results throw no light on the problem of which theory to choose. All the thermodynamic relations we have deduced hold on any theory of solution and favour no one theory rather than another. Whether osmotic pressure be due to physical impact or to chemical affinity it must necessarily have the gas value in a dilute solution, and be related to vapour pressure and freezing point in the way we have traced. But for any theory of solution to be tenable, it must at least be consistent with the known thermo- dynamic relations, verified as those relations are by experiment.
On certain assumptions required for the extension of the methods of the kinetic theory of gases to liquids, L. Boltzmann offered a demonstration of the law of osmotic pressure in dilute
solutions, based on the idea that the mean energy of translation of a molecule should be the same in the liquid as in the gaseous state. But, whether or not the assumption underlying this demonstration be accepted, the similarity between solution and chemical action remains, and the osmotic law has been examined from this side by J. H. Poynting and by H. L. Callendar. The fundamental phenomenon they take to be the identity of vapour pressure, and consider the combination necessary to reduce the vapour pressure of a solution to the right value. If each mole- cule of the solute combines with a certain number of molecules of the solvent in such a way as to render them inactive for evapora- tion, we get a lowering of vapour pressure. Let us assume that the ratio pjp' of the vapour pressures of the solvent and solution is equal to the ratio of the number of free molecules of solvent to the whole number of molecules in the solution. Each molecular complex, formed by solution and solvent, is treated as a single molecule. If there are n molecules of solute to N of solvent originally, and each molecule of solute combines with a molecule of solvent, we get for the ratio of vapour pressures pjp' — (N — a«)/(N — a»+»), while the relative lowering of vapour pressure is (p — p')lp = nj(N — an).
In the limit of dilution when n is very small compared with N this gives Raoult's experimental law that the relative lowering is w/N, which we deduced from the osmotic law, and conversely from which the osmotic law follows, while for more concentrated solutions agreement is obtained by assigning arbitrary values to a, which, as we have seen, is 5 in the case of cane-sugar.
Certain solvents, such as water, liquid ammonia or liquid hydrocyanic acid, possess the power of making some solutes, such as mineral salts and acids, when dissolved in them, con- ductors of electricity. The special properties of these solutions are dealt with under Electrolysis and Conduction, Elec- tric, § In Liquids. Attempts have been made to co-ordinate this ionizing power of solvents with their dielectric constants, or with their chemical properties. On the lines of Poynting's theory of solution, each ion in electrolytes must combine with one or more molecules of solvent.
Diffusion in Solutions. — The passage of dissolved substances through animal and vegetable membranes was the subject of many early experiments. It was found that substances like mineral salts, which crystallize well from solution, passed such membranes with comparative ease, while the jelly-like substances such as albumen passed with extreme slowness if at all. The first to make systematic experiments on the free diffusion of dissolved substances with no separating membrane was Thomas Graham (1804-1860), who immersed in a large volume of water a wide-mouthed bottle containing a solution, and after some time measured the quantity of substance which had diffused into the water. Again the two classes of substances mencioned above were found to be distinguished, and Graham called the slowly diffusible non-crystalline bodies colloids, in contrast to the quickly diffusible crystalloids. Graham showed that the diffusion was approximately proportional to the difference in con- centration, and on these lines a theory of diffusion was founded on the lines of Fourier's treatment of the conduction of heat.
The quantity of substance which diffuses through unit area in one second may be taken as proportional to the difference in. con- centration between the fluids at that area and at another parallel area indefinitely near it. This difference in concentration is proportional to the rate of variation — dc/dx of the concentration c with the distance x, so that the number of gramme-molecules of solute which, in a time dt, cross an area A of a long cylinder of. constant cross section is dN = —DA(dc/dx)dt, where D is a constant known as the diffusion constant or the diffusivity.
The osmotic pressure of a solution depends on the concentration; and, if we regard the difference in that pressure as the effective force driving the dissolved substance through the solution, we are able to obtain the equation of diffusion in another form. When the solution is dilute enough for the osmotic pressure to possess " the gas " value the equation becomes —
dN '= ~^r A dl dt '
where R is the usual gas constant, T the absolute temperature,
and F the force required to drive one gramme-molecule of the solute through the solution with unit velocity. By comparison with the first equation we see that RT/F is equal to D, the diffusion constant. This constant can be measured experimentally, and for such a substance as sugar or water comes out about 0.3 at 20° C, the unit of time being the day. Hence the force required to drive one gramme-molecule of sugar through water with a velocity of one centimetre per second may be calculated as some thousands of millions of kilogrammes weight.
In the case of electrolytes we can go further, and calculate the diffusion constant itself from the theory of electrolytic dissociation (see Conduction, Electric, § In Liquids). On that theory the ions of a dilute solution migrate independently of each other. Since some ions are more mobile than others, a separation will ensue when water is placed in contact with a solution, the faster moving ion penetrating quicker into the water under the driving force of the osmotic pressure gradient. This separation causes a difference of potential, which can be calculated and is found to agree with the values obtained experimentally. The separation also sets up electrostatic forces, which increase until they are strong enough to drag the slower moving ions along faster, and to retard the naturally faster ions till they travel at the same rate. The resistance offered by the liquid, and therefore the force F, required to drive one gramme-molecule through the liquid with unit velocity is the sum of the corresponding quantities for the individual ions. Now the velocities u and v of the opposite ions under unit potential gradient, and therefore U and V under unit force, are known from electrical data. Thus F, which is equal to 1/U + 1/V, is known. The osmotic pressure of an electrolyte consisting of two ions is double that of a non-electrolyte. Hence for a binary electrolyte the diffusion constant is measured by 2RT/F or 2UVRT/(U+V). This result gives a value of D for dilute hydrochloric acid equal to 2.49 to compare with the observed value of 2.30. Other substances give equally good agreements; thus sodium chloride has a calculated constant of 1.12 and an observed one of 1.11. Such concordance gives strong support to the theory of diffusion outlined above.
Colloidal Solutions.—Besides a large number of animal and vegetable substances, many precipitates formed in the course of inorganic chemical reactions are non-crystalline and appear in the colloidal state, instances are the sulphides of antimony and arsenic and the hydroxides of iron and alumina. Some of these colloids dissolve in water or other liquids to form solutions called by Graham hydrosols; Graham named the solids formed by the setting or coagulation of these liquids hydrogels. Solutions of colloids in solvents such as water and alcohol seem to be divisible into two classes. Both mix with warm water in all proportions, and will solidify in certain conditions. One class, represented by gelatin, will redissolve on warming or diluting, while the other class, containing such substances as silica, albumen, and metallic, hydrosulphides, will solidify on heating or on the addition of electrolytes to form a solid “gel” which cannot be redissolved. Solidification of the first kind may be termed “setting,” that of the second “coagulation.”
The power of coagulation of colloids shown by electrolytes depends in a curious manner on the chemical valency of the effective ion. The average of the coagulative powers of salts of univalent, divalent and trivalent metals have been found by experiment to be proportional to the numbers 1: 35 : 1023. If we assume that a certain minimum electric charge must be brought into contact with a group of colloid particles to produce coagulation, twice as many univalent ions must collect to produce the same effect as a number of divalent ions, and three times as many as an effective number of trivalent ions. We can calculate, by the help of the kinetic theory and the theory of chances, the frequency with which the necessary conjunctions of ions will occur, and show that the general law will be that the coagulative powers should be in the ratios of 1 : x : x2 . Putting x = 32, we get 1 : 32 : 1024 to compare with the experimental numbers. The ordinary surface energy of a two-phase system tends to diminish the area of contact, and thus to help the growth of the larger aggregates required for coagulation. A natural electric charge on the particles would oppose this tendency, and tend to increase the free surface and thus promote disintegration and solution. The function of the electrolyte may be to annul such a natural charge and thus allow the non-electric surface energy to produce coagulation. This explanation is supported by some experiments by W. B. Hardy, who found that certain colloids did possess electric charges, the sign of which depended on whether the surrounding liquid was slightly acid or slightly alkaline. At the neutral point, when the particles possessed no charge, their stability was destroyed, and they were precipitated. But recent experiments have shown that the simple theory of coagulation here outlined needs amplification in certain directions. The phenomena seem to be dependent on variables such as time, and are more complicated than seemed likely at first.
The size of the suspended particles in colloidal solutions varies greatly. In some solutions they are visible under a good microscope. In other cases, while too small to be directly visible, they are large enough to scatter and polarize a beam of light. In yet other solutions, the particles are smaller again, and seem to approach in size the larger molecules of crystalloid substances. It is not yet agreed whether colloid solution is the same in kind though different in degree from crystalloid solution or is a phenomenon of an entirely different order.
References.—The properties and theory of solutions are treated in all works on general physical chemistry; Ostwald’s discussion in his Lehrbuch was translated into English in 1891 by M. M. P. Muir entitled Solution. Special works are W. C. D. Whetham, Theory of Solution (1902); W. Rothmund, Löslichkeit (1907). Solubility tables are given in Landolt, Börnstein and Meyerhoffers, Tabellen (1905); A. M. Comey, Dictionary of Solubilities (Inorganic) (1896); A. Seidell, Dictionary of the Solubilities of Inorganic and Organic Substances (1907). (W. C. D. W.)
