Aviation Weather AC 00-6A/The Earth's Atmosphere

Chapter 1: The Earth's Atmosphere
[edit]Planet Earth is unique in that its atmosphere sustains life as we know it. Weather—the state of the atmosphere—at any given time and place strongly influences our daily routine as well as our general life patterns. Virtually all of our activities are affected by weather, but of all man's endeavors, none is influenced more intimately by weather than aviation.
Weather is complex and at times difficult to understand. Our restless atmosphere is almost constantly in motion as it strives to reach equilibrium. These never-ending air movements set up chain reactions which culminate in a continuing variety of weather. Later chapters in this book delve into the atmosphere in motion. This chapter looks briefly at our atmosphere in terms of its composition; vertical structure; the standard atmosphere; and of special concern to you, the pilot, density and hypoxia.
Composition
[edit]
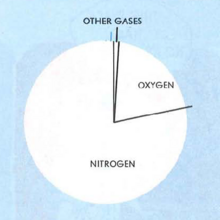
Air is a mixture of several gases. When completely dry, it is about 78% nitrogen and 21 % oxygen. The remaining 1% is other gases such as Argon, Carbon Dioxide, Neon, Helium, and others. Figure 1 graphs these proportions. However, in nature, air is never completely dry. It always contains some water vapor in amounts varying from almost zero to about 5% by volume. As water vapor content increases, the other gases decrease proportionately.
Vertical Structure
[edit]

We classify the atmosphere into layers, or spheres, by characteristics exhibited in these layers. Figure 2 shows one division which we use in this book. Since most weather occurs in the troposphere and since most flying is in the troposphere and stratosphere, we restrict our discussions mostly to these two layers.
The troposphere is the layer from the surface to an average altitude of about 7 miles. It is characterized by an overall decrease of temperature with increasing altitude. The height of the troposphere varies with latitude and seasons. It slopes from about 20,000 feet over the poles to about
65,000 feet over the Equator; and it is higher in summer than in winter. At the top of the troposphere is the tropopause, a very thin layer marking the boundary between the troposphere and the layer above. The height of the tropopause and certain weather phenomena are related. Chapter 13 discusses in detail the significance of the tropopause to flight. Above the tropopause is the stratosphere. This layer is typified by relatively small changes in temperature with height except for a warming trend near the top.
The Standard Atmosphere
[edit]Continual fluctuations of temperature and pressure in our restless atmosphere create some problems for engineers and meteorologists who require a fixed standard of reference. To arrive at a standard, they averaged conditions throughout the atmosphere for all latitudes, seasons, and altitudes. The result is a standard atmosphere with specified sea-level temperature and pressure and specific rates of change of temperature and pressure with height. It is the standard for calibrating the pressure altimeter and developing aircraft performance data. We refer to it often throughout this book.
Density and Hypoxia
[edit]Air is matter and has weight. Since it is gaseous, it is compressible. Pressure the atmosphere exerts on the surface is the result of the weight of the air above. Thus, air near the surface is much more dense than air at high altitudes. This decrease of density and pressure with height enters frequently into our discussions in later chapters.
The decrease in air density with increasing height has a physiological effect which we cannot ignore. The rate at which the lungs absorb oxygen depends on the partial pressure exerted by oxygen in the air. The atmosphere is about one-fifth oxygen, so the oxygen pressure is about one-fifth the total pressure at any given altitude. Normally, our lungs are accustomed to an oxygen pressure of about 3 pounds per square inch. But, since air pressure decreases as altitude increases, the oxygen pressure also decreases. A pilot continuously gaining altitude or making a prolonged flight at high altitude without supplemental oxygen will likely suffer from hypoxia—a deficiency of oxygen. The effects are a feeling of exhaustion; an impairment ofvision and judgment; and finally, unconsciousness. Cases are known where a person lapsed into unconsciousness without realizing he was suffering the effects.
When flying at or above 10,000 feet, force yourself to remain alert. Any feeling of drowsiness or undue fatigue may be from hypoxia. If you do not have oxygen, descend to a lower altitude. If fatigue or drowsiness continues after descent, it is caused by something other than hypoxia.
A safe procedure is to use auxiliary oxygen during prolonged flights above 10,000 feet and for even short flights above 12,000 feet. Above about 40,000 feet, pressurization becomes essential.
