at constant volume. This necessitates the application of a stem-exposure correction, the value of which is approximately given by the formula
| dt=rt(t−100)/T2, | (11) |
where r is the ratio of the volume of the dead space to the volume of the thermometric bulb, and T2 is the mean temperature of the dead space, which is supposed to be constant. The magnitude of the correction is proportional to the ratio r, and increases very rapidly at high temperatures. If the dead space is 1 per cent. of the bulb, the correction will amount to only one-tenth of a degree at 50° C., but reaches 5 ° at 445 °C., and 30° at 1000 °C. It is for this reason important in high-temperature work to keep the dead space as small as possible and to know its volume accurately. With a mercury manometer, the volume is liable to a slight uncertainty on account of changes of shape in the meniscus, as it is necessary to use a wide tube in order to secure accurate measurements of pressure.
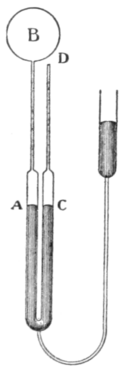 |
| Fig. 4.—Method of Compensation. |
13. Compensation Method with Oil-Gauge.—It is possible to avoid this difficulty, and to make the dead space very small, by employing oil or sulphuric acid or other non-volatile liquid to confine the gas in place of mercury (Phil. Trans., A. 1887, p. 171). The employment of a liquid which wets the tube makes it possible to use a much smaller bore, and also greatly facilitates the reading of small changes of pressure. At the same time the instrument may be arranged so that the dead space correction is automatically eliminated with much greater accuracy than it can be calculated. This is effected as shown diagrammatically in fig. 4, by placing side by side with the tube AB, connecting the bulb B to the manometer A, an exact duplicate CD, closed at the end D, and containing liquid in the limb C, which is of the same size as the branch A of the manometer and in direct communication with it. The tube CD, which is called the compensating tube, contains a constant mass of gas under exactly similar conditions of volume and temperature to the tube AB. If therefore the level of the liquid is always adjusted to be the same in both tubes AB and CD, the mass of gas contained in the dead space AB will also be constant, and is automatically eliminated from the equations, as they contain differences only.
14. Gravimetric Method.—In the writer's opinion, the gravimetric or overflow method, although it has seldom been adopted, and is not generally regarded as the most accurate, is much to be preferred to the manometric method, especially for work at high temperatures. It is free from the uncertain corrections above enumerated as being peculiar to the manometric method. The apparatus is much simpler to manipulate and less costly to construct. If the pressure is kept constant and equal to the external atmospheric pressure, there is no strain of the bulb, which is particularly important at high temperatures. There is no dead space correction so long as the temperature of the dead space is kept constant. The troublesome operation of reading and adjusting the mercury columns of the manometer is replaced by the simpler and more accurate operation of weighing the mercury displaced, which can be performed at leisure. The uncertain correction for the temperature of the mercury in the manometer is entirely avoided.
The reasons which led Regnault to prefer the constant-volume thermometer are frequently quoted, and are generally accepted as entirely conclusive, but it is very easy to construct the constant-pressure or gravimetric instrument in such a manner as to escape the objections which he urges against it. Briefly stated, his objections are as follows: (1) Any error in the observation of the temperature of the gas in the overflow space produces a considerable error in the temperature deduced, when the volume of the overflow is large. This source of error is very simply avoided by keeping the whole of the overflow in melting ice, an expedient which also considerably simplifies the equations. It happened that Regnault’s form of thermometer could not be treated in this manner, because he had to observe the level of the mercury in order to measure. the pressure and the volume. It is much better, however, to use a separate gauge, containing oil or sulphuric acid, for observing small changes of pressure. The use of ice also eliminates the correction for the variation of density of the mercury by which the overflow is measured. (2) Regnault's second objection was that an error in the measurement of the pressure, or in reading the barometer, was more serious at high temperatures in the case of the constant-pressure thermometer than in the constant-volume method. Owing to the incessant variations in the pressure of the atmosphere, and in the temperature of the mercury columns, he did not feel able to rely on the pressure readings (depending on observations of four mercury surfaces with the cathetometer) to less than a tenth of a millimetre of mercury, which experience showed to be about the limit of accuracy of his observations. This would be equivalent to an error of 0·036° with the constant-volume thermometer at any point of the scale, but with the constant-pressure thermometer the error would be larger at higher temperatures, since the pressure does not increase in proportion to the temperature. This objection is really unsound, because the ideal condition to be aimed at is to keep the proportionate error dT/T constant. That the proportionate error diminishes with rise of temperature, in the case of the constant-volume thermometer, is really of no advantage, because we can never hope to be able to measure high temperatures with greater proportionate accuracy than ordinary temperatures. The great increase of pressure at high temperatures in the manometric method is really a serious disadvantage, because it becomes necessary to work with much lower initial pressures, which implies inferior accuracy at ordinary temperatures and in the determination of the initial pressure and the fundamental interval.
15. Compensated Differential Gas Thermometer.—The chief advantage of the gravimetric method, which Regnault and others appear to have missed, is that it is possible to make the measurements altogether independent of the atmospheric pressure and of the observation of mercury columns. This is accomplished by using, as a standard of constant pressure, a bulb S, fig. 5, containing a constant mass of gas in melting ice, side by side with the bulb M, in which the volume of the overflow is measured. The pressure in the thermometric bulb T is adjusted to equality with the standard by means of a delicate oil-gauge G of small bore, in which the difference of pressure is observed by means of a cathetometer microscope. This kind of gauge permits the rapid observation of small changes of pressure, and is far more accurate and delicate than the mercury manometer. The fundamental measurement of the volume of the overflow in terms of the weight of mercury displaced at 0°C. involves a single weighing made at leisure, and requires no temperature correction. The accuracy obtainable at ordinary temperatures in this measurement is about ten times as great as that attainable under the best conditions with the mercury manometer. At higher temperatures the relative accuracy diminishes in proportion to the absolute temperature, or the error dt increases according to the formula
| dt/t =− (T/T0) dw/w, | (12) |
where w is the weight of the overflow and dw the error. This diminution of the sensitiveness of the method at high temperatures is commonly urged as a serious objection to the method, but the objection is really without weight in practice, as the possible accuracy of measurement is' limited by other conditions. So far as the weighing alone is concerned, the method is sensitive to one-hundredth of a degree at 1000°C., which is far beyond the order of accuracy attainable in the application of the other corrections.
16. Method of Using the Instrument.—A form of gas thermometer constructed' on the principles above laid down, with the

