| i | Latent heat of water vapor[1] ( = deg. fahr.) |
| j | Instantaneous specific heat of water vapor (approximately)
|
| k | Specific weight of water vapor at saturation for any pressure and temperature
|
43With respect to the reliability of these data, those on the latent heat of steam may be accepted as absolute within 0.1 per cent,
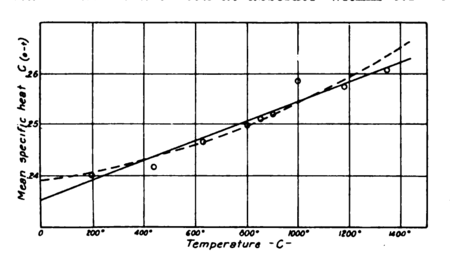
since the agreement of recent investigators seems to have established the present values beyond question. Strange to say, however, the specific heat of air, as already pointed out, has not been established with accuracy within 2 per cent. Regnault gives it as a constant, , and this value has generally been accepted. However, Holborn and Henning, whose valuable determinations in steam are well known, have demonstrated it to be a variable. For nitrogen they give a value ( in deg. cent.), a straight-line relationship, although for superheated vapors they find equations of a higher degree. The plot of their values for for nitrogen, as shown in Fig. 7, lacks considerable uniformity. So far as the points given are concerned they do not seem to warrant assuming a straight-line relationship. Their points would seem to indicate rather a curve with considerably greater values at lower temperatures
- ↑ C. H. Peabody, Trans.Am.Soc.M.E., vol. 31. p. 334, 1909.