eliminated below the amount required in steel. As stated, this problem was solved by burning out practically all of the carbon and then adding a definite amount in the form of a recarburizer. In the same way, the necessary quantity of manganese was supplied. The success of the Bessemer process was not established commercially until 1860; from that date it has grown by leaps and bounds, until to-day it is perhaps the most important of the steel-making processes. Bessemer reaped fame and wealth from his invention, of which it has been truly remarked that it was of far more importance to the world than all the gold of California and Australia.
The open-hearth process of steel-making consists in making pig iron mixed with a greater or less quantity of wrought iron, steel, or similar iron products by exposure to the direct action of flame in a regenerative gas-furnace, and converting the resultant bath into fluid steel. Like the Bessemer process, the open-hearth process is divided into an acid process and a basic process; in the first the hearth is lined with sand and the slag is siliceous, and in the second the hearth is lined with a basic material and the slag is basic. Like the Bessemer process, also, the consideration of the open-hearth process may be divided into a discussion, first of the plant and mechanical operations, and then of the chemical reactions.
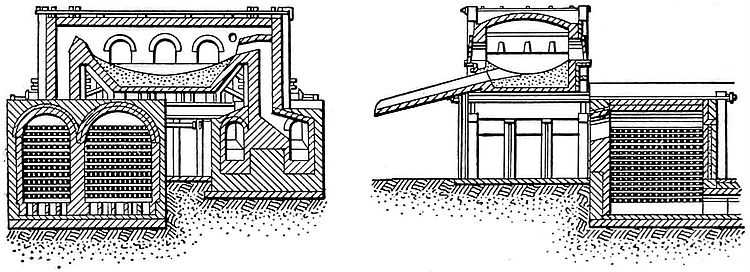
The central figure of an open-hearth steel plant is the melting-furnace. This is always a regenerative furnace, for in no other construction is it practicable to obtain the necessary temperature, but its construction varies greatly in other respects. Fig. 3, showing a furnace designed for the Illinois Steel Company, of Chicago, may be used to explain the general features of construction and operation of an open-hearth steel furnace. In this drawing the checker-work shows the regenerating chambers, and the saucer-shaped structure above is the hearth. The two regenerating chambers are separate from each other, but either may be connected with the hearth and with the smokestack. Each chamber is nearly filled with a sort of cob-house work of brick. In operation, one chamber is heated so that the brick filling is white hot, and then a current of air and gas is admitted at the bottom. As this combustible mixture passes up through the heated brickwork, it is heated so that when it arrives at a point over the hearth it is burning fiercely. A portion of the heat of this combustion is given up to the charge on the hearth. From the hearth the hot gases pass down through the brickwork of the second chamber and serve to heat it, after which they escape at the bottom into the smokestack flue. As soon as the brickwork of the first chamber has become cooled below a certain point, the current of gases is reversed, so that they enter at the bottom of the second chamber and pass out of the bottom of the first chamber after being heated and ignited by the hot brickwork through which they have passed, and after passing over the hearth giving up their waste heat to the brickwork of the first chamber. By repetitions of this process of reversing the current of gases, the regenerative process is continuous, and a steady, intense heat is maintained on the hearth.
The hearth is usually a fixed structure, as shown by Fig. 3, but in some American works it is so constructed that it can be tilted like a Bessemer converter to receive its charge and to discharge its molten contents. This form of hearth is claimed to have several material advantages over the fixed hearth in case of operation and in producing steel from certain materials for certain special purposes. Whether fixed or tilting, the construction of the hearth consists of a steel and iron plate shell lined with some refractory material. In hearths for the acid process, the hearth-lining proper consists first of a layer of sand fused into a solid mass by heat. In hearths for the basic process the lining consists of a plastic compound made by roasting and grinding dolomite limestone and mixing the powder with tar. The accessories to the hearth consist of charging and tapping appliances, ladles and cranes for handling them, casting molds, etc.
In respect to their chemistry, the acid and the basic open-hearth process can best be considered separately. Referring first to the acid process, the proportions of the constituents of the charge vary in different places. Sometimes pig iron alone is used, but more generally pig iron and scrap wrought iron and steel are mixed. Whatever the mixture may be, it is necessary that its contents of phosphorus and sulphur be known, since these are not eliminated in the process. Broadly considered, the chemical reactions may be divided into three operations: first, the reactions during melting; second, the reactions after melting; and third, the reactions during recarburization. During melting the silicon and manganese are reduced by oxidization to a minute fraction of their original amounts and