Popular Science Monthly/Volume 56/January 1900/The Applications of Explosives I
| THE APPLICATIONS OF EXPLOSIVES. |
By CHARLES E. MUNROE,
PROFESSOR OF CHEMISTRY, COLUMBIAN UNIVERSITY.
THERE is something about fire which fascinates every one, yet the action of explosives arouses even a livelier interest, since the accompanying fiery phenomena are more intense and are attended with a shocking report and a violent destruction of the surrounding material, while this train of events, with all its marked

Gun-Cotton Factory. Dipping cotton in nitrating troughs.

Gin-Cotton Factory. Digestion troughs.
the explosive be properly placed in well-proportioned amounts and discharged at the right time, can be made to do useful and important work that can not be as conveniently and quickly accomplished in most cases, and in some cases can not be accomplished at all by any other means.
The marked characteristic of all explosive substances, and especially of the so-called high explosives, is that the energy, as developed, is at high potential, and the uses to which energy in this condition can be economically put are so manifold that the production of explosives has become one of the most important of our chemical industries, this country alone producing, in 1890, 108,735,980 pounds, having a value of nearly $11,000,000.
The number of possible substances possessing explosive properties is exceedingly large; the number actually known is so great that it has taxed the ingenuity of inventors to provide them with suitable names; but these various explosive substances vary to so great an extent in the energy they will develop in practice and in their safety in storage, transportation, and use that but a comparatively small number have met with wide acceptance. All may be classified under the heads of physical mixtures like gunpowder, or chemical compounds like nitroglycerin, and they owe their development of energy to the fact that, like gunpowder, they are mixtures in which combustible substances such as charcoal are mixed with supporters of combustion such as niter; or that, like chloride of nitrogen, they are chemical compounds, the formation of whose molecules is attended with the absorption of heat; or that, like gun cotton, they are chemical compounds whose molecules contain both the combustible and the supporter of combustion, and whose formation from their elements is attended with the absorption of heat; while occupying a middle place between the gunpowder and the

Gun-Cotton Factory. Final press.
gun-cotton class, and possessing also to some degree the properties of the nitrogen-chloride class, are the nitro-substitution explosives, of which melinite, emmonsite, lyddite, and joveite furnish conspicuous examples.
It may lead to a clearer understanding of what is said regarding the applications of explosives to dwell briefly on the methods by which some of them are produced, since, although the raw material in each case is different and the details of the operations vary, the underlying principles of the methods are the same, and a good example is found in the military gun cotton as made by the Abel process at the United States Naval Torpedo Station. The material employed is cotton, but whether fresh from the field or in the form of waste, it must first be freed from dirt by hand picking and sorting, and from grease  Gunpowder Grains. The large ones are over five pounds weight, each. and incrusting substances by boiling in a weak soda solution. The cotton is now dried by wringing in a centrifugal wringer and exposing to a current of hot air in a metal closet; but as the compacted mass of cotton holds moisture with great persistency, after partial drying the cotton is passed through a cotton picker to open the fiber, so that it not only yields its contained water more readily and completely, but it also absorbs the acids more speedily in the dipping process to which it is subsequently exposed.
Gunpowder Grains. The large ones are over five pounds weight, each. and incrusting substances by boiling in a weak soda solution. The cotton is now dried by wringing in a centrifugal wringer and exposing to a current of hot air in a metal closet; but as the compacted mass of cotton holds moisture with great persistency, after partial drying the cotton is passed through a cotton picker to open the fiber, so that it not only yields its contained water more readily and completely, but it also absorbs the acids more speedily in the dipping process to which it is subsequently exposed.
When the moisture, by the final drying, is reduced to one half of one per cent the cotton is, while hot, placed in copper tanks which close hermetically, where it cools to the atmospheric temperature and in which it is transported to the dipping room, where a battery of large iron troughs, filled with a mixture of one part of the most concentrated nitric acid and three parts of the most concentrated sulphuric acid, set in a large iron water bath to keep the mixture at a uniform temperature, is placed under a hood against the wall. The fluffy cotton, in one-pound lots, is dipped handful by handful under the acid, by means of an iron fork, where it is allowed to remain for ten minutes, when it is raised to the grating at the rear of the trough and squeezed with the lever press to remove
 | ||
| Burning Disk of Gun Cotton. | Extinguishing burning Gun Cotton. | |
the excess of acid. It still retains about ten pounds of the acid mixture, and in this condition is placed in an acid-proof stoneware crock, where it is squeezed by another iron press to cause the contained acid to rise above the surface of the partly converted cotton. The covered crock is now placed with others in wooden troughs containing running water so as to keep the temperature uniform, where the cotton is allowed to digest for about twenty-four hours. The acid is then wrung out in a steel centrifugal, and the wrung gun cotton is thrown in small lots into an immersion tank containing a large volume of flowing water, in which a paddle wheel is revolving so as to rapidly dilute and wash away the residual

Making Mercury Fulminate.
acid in the gun cotton without permitting any considerable rise of temperature from the reaction of the water with the acid.
Even these severe means are not enough, for, as the cotton fiber is in the form of hairlike tubes, traces of the acid sufficient to bring about the subsequent decomposition of the gun cotton are retained by capillarity. Therefore, after boiling with a dilute solution of sodium carbonate, the gun cotton is pulped and washed in a beater or rag engine until the fiber is reduced to the fineness of corn meal, and a sample of it will pass the "heat test." This is a test of the resistance of gun cotton to decomposition, and requires that when the ![]() Detonator used in the United States Navy.
Detonator used in the United States Navy.
Contains thirty-five grains of fulminate of mercury. air-dried sample of gun cotton is heated to 65.5° C in a closed tube in which a moistened strip of potassium iodide and starch paper is suspended, the paper should not become discolored in less than fifteen minutes' exposure.
This pulping of the gun cotton not only enables one to more completely purify it, but it also renders it possible to mold it into convenient forms and to compress it so as to greatly increase its efficiency in use. For this purpose the pulp is suspended in water

Torpedo Cases and Blocks of Wood destroyed by a Naval Detonator.
and pumped to a molding press, where, under a hydraulic pressure of one hundred pounds to the square inch, it is molded into cylinders or prisms about three inches in diameter and five inches and a half high, and these are compressed to two inches in height by a final press exerting a pressure of about sixty-eight hundred pounds to the square inch. As this is regarded as a somewhat hazardous operation, the press is surrounded by a mantlet woven from stout rope to protect the workmen from flying pieces of metal in case of an accident. The operation is analogous to that  Testing Detonators on Iron Plates. employed in powder-making, where the gunpowder has been pressed in a great variety of forms and into single grains weighing several pounds apiece.
Testing Detonators on Iron Plates. employed in powder-making, where the gunpowder has been pressed in a great variety of forms and into single grains weighing several pounds apiece.
Even under the enormous pressure of the final press the compressed gun cotton still retains from twelve to sixteen per cent of water, and in this form it is quite safe to store and handle. When dry it is very combustible and burns readily when ignited, but it can be quenched by pouring water upon it. When confined in the chamber of a gun or the bore-hole of a rock, gun cotton will burn like gunpowder when ignited, if dry, and produce an explosion, but, in common with nitroglycerin  Iron Cylinder filled with Water and containing A Naval Detonator. Before and after firing, shows the work accomplished by thirty-five grains of mercury fulminate. and other high explosives, gun cotton is best exploded and develops its maximum effect when detonated, a result which is secured by exploding a small quantity of mercury fulminate in contact with the dry material.
Iron Cylinder filled with Water and containing A Naval Detonator. Before and after firing, shows the work accomplished by thirty-five grains of mercury fulminate. and other high explosives, gun cotton is best exploded and develops its maximum effect when detonated, a result which is secured by exploding a small quantity of mercury fulminate in contact with the dry material.
Mercury fulminate is made by dissolving mercury in nitric acid and pouring the solution thus produced into alcohol, when a violent reaction takes place and the fulminate is deposited as a crystalline gray powder. This powder is loaded in copper cases and, after drying, it is primed with dry-mealed

Smokeless Powders. In the bottle is indurite in flake grains. The larger grains are cylindrical and hexagonal multiperforated United States army grains. The bent grain in the foreground, looking like a piece of rubber tubing, is a grain of Maxim powder with a single canal. The flat strips in the foreground on the left are grains of the French B. N. powder. The flat strips in the foreground on the right are grains of the United States navy "pyrocellulose" powder.
gun cotton, the mouth of the case being closed with a sulphur-glass plug, through which pass two copper leading wires joined by a bridge of platinum-iridium wire, two one-thousandths of an inch in diameter, which becomes heated to incandescence when an electric current is sent through it. This device is what is known as the naval detonator. Mercury fulminate is so employed because it is the most violent
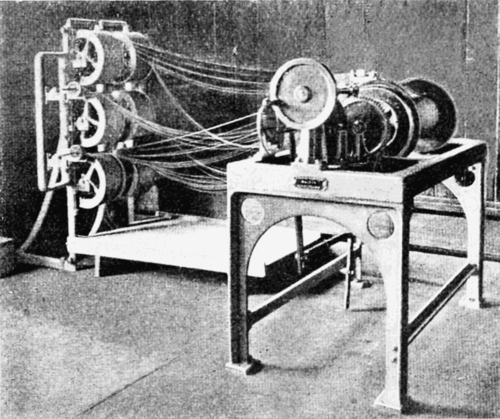
Blending Machine for Cordite.
of all explosives in common use, and exerts a pressure of forty-eight thousand atmospheres when fired in contact. Although the naval detonator contains but thirty-five grains of mercury fulminate, yet it will rupture stout iron and heavy tin torpedo cases when fired suspended in them, it will rend thick blocks of wood when placed in a hole and fired within them, and it will even pierce holes through plates of the finest wrought iron one-sixteenth inch in thickness if only the base of the detonator is in contact with the plate, and this has been used as a test of their efficiency. Its force is markedly shown by firing one in a stout iron cylinder filled with water and closed tightly, when the cylinder is blown into a shredded sphere. When used to detonate gun cotton, either when confined or in the open, the detonator is placed in the hole which has been molded in the center of the gun-cotton disk or block, so that it shall be in close contact with the gun cotton.  Cartridge of Cordite Smokeless Powder. Charge for 6-inch 2 F gun, 13 pounds, 4 ounces. Cords, 223⁄4 inches long, 3 inches in diameter. I have found that perfectly dry compressed gun cotton is detonated by 2.83 grains of mercury fulminate; but as a torpedo attack is necessarily in the nature of a forlorn hope and should be provided with every possible provision against failure, and since if the detonator fails the attack fails, the naval detonator is supplied with thirty-five grains, so as to give a large coefficient of assurance. A characteristic feature of gun cotton is that it may be detonated even when completely saturated with and immersed in water,
if only some dry gun cotton be detonated in contact with it.
Cartridge of Cordite Smokeless Powder. Charge for 6-inch 2 F gun, 13 pounds, 4 ounces. Cords, 223⁄4 inches long, 3 inches in diameter. I have found that perfectly dry compressed gun cotton is detonated by 2.83 grains of mercury fulminate; but as a torpedo attack is necessarily in the nature of a forlorn hope and should be provided with every possible provision against failure, and since if the detonator fails the attack fails, the naval detonator is supplied with thirty-five grains, so as to give a large coefficient of assurance. A characteristic feature of gun cotton is that it may be detonated even when completely saturated with and immersed in water,
if only some dry gun cotton be detonated in contact with it.  Gun-Cotton Spar Torpedo. Thus in one experiment a disk of dry gun cotton was covered with a water-proof coating and the detonator inserted in the detonator hole of this disk. This dry disk was laid upon four uncoated disks, the five lashed tightly together, and sunk in Newport Harbor, where the column remained until the uncoated disks were saturated with salt water, when the mine was fired and the saturated disks were found by measurement of the work done to have been completely exploded. I have found that three ounces of dry compressed gun cotton v/ill cause the detonation of wet compressed gun cotton in contact with it, but forty ounces of dry gun cotton are used as the primer in our naval mines and torpedoes, so as to give a large coefficient of assurance.
Gun-Cotton Spar Torpedo. Thus in one experiment a disk of dry gun cotton was covered with a water-proof coating and the detonator inserted in the detonator hole of this disk. This dry disk was laid upon four uncoated disks, the five lashed tightly together, and sunk in Newport Harbor, where the column remained until the uncoated disks were saturated with salt water, when the mine was fired and the saturated disks were found by measurement of the work done to have been completely exploded. I have found that three ounces of dry compressed gun cotton v/ill cause the detonation of wet compressed gun cotton in contact with it, but forty ounces of dry gun cotton are used as the primer in our naval mines and torpedoes, so as to give a large coefficient of assurance.
In the mining and other industries the fulminate is used in smaller quantities and it is generally mixed with potassium chlorate, the mixture being compressed in small copper cases  Blowing up the Schooner Joseph Henry. and sold as blasting caps. They are fired by means of a piece of Bickford or running fuse, consisting of a woven cotton or hemp tube containing a core of gunpowder, which is inserted in the mouth of the copper cap and made fast within it by crimping. The capped fuse is then inserted in a dynamite cartridge so that the cap is firmly in contact with the dynamite, the mouth of the cartridge is fastened securely, and the charge inserted in the borehole in the rock and tamped, The protruding end of the fuse is lighted, and the fire travels at the rate of three feet per minute down the train of gunpowder to the fulminate, which then detonates and causes the detonation of the dynamite.
Blowing up the Schooner Joseph Henry. and sold as blasting caps. They are fired by means of a piece of Bickford or running fuse, consisting of a woven cotton or hemp tube containing a core of gunpowder, which is inserted in the mouth of the copper cap and made fast within it by crimping. The capped fuse is then inserted in a dynamite cartridge so that the cap is firmly in contact with the dynamite, the mouth of the cartridge is fastened securely, and the charge inserted in the borehole in the rock and tamped, The protruding end of the fuse is lighted, and the fire travels at the rate of three feet per minute down the train of gunpowder to the fulminate, which then detonates and causes the detonation of the dynamite.
Although gun cotton, nitroglycerin, and their congeners can be and usually are fired by detonation, there has within recent years been a great number of compositions invented which, while formed from gun cotton alone or mixtures of it with nitroglycerin, burn progressively when ignited and are therefore available for use as propellants; and since the products of their burning are almost wholly gaseous, they produce but little or no smoke and are therefore called smokeless powders. As upward of fifty-seven per cent of the products of the burning of ordinary gunpowder are solids or easily compressed vapors, this comparative smokelessness of the modern powders is a very important characteristic, and
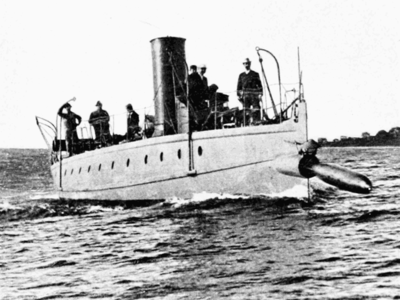
Torpedo Practice. Bow discharge.
when used in battle they seriously modify our former accepted methods of handling troops. While this is the feature of these powders which has attracted popular attention, a far more important quality which they possess is the power to impart to a projectile a much higher velocity than black powder does, without exerting an undue pressure on the gun. A velocity of over twenty-four hundred feet per second has been imparted to a one-hundred pound projectile with the powder that I have invented for our navy, while the pressure on the gun was less than fifteen tons to the square inch.
Prior to my work in this field all the so-called smokeless powders were mixtures of several ingredients, resembling gunpowder in this respect. But, considering the precise and difficult work that was expected of these high-powered powders and the difficulty which had always been found in securing uniformity in mixtures, and that this difficulty had become the more apparent as the gun became more highly developed, I sought to produce a powder which should consist of a single chemical substance in a state of chemical purity, and which could be formed into grains of such form and size as were most suitable for the piece in which the powder was to be used.
I succeeded in so treating cellulose nitrate of the highest degree of nitration as to convert it into a mass like ivory and yet leave it pure. In this indurated condition the gun cotton will burn freely, but it has not been possible to detonate it even when closely confined and exposed to the initial detonation of large masses of mercury fulminate.

Torpedo Practice on the Cushing. Broadside discharge.
I am happy to say that this principle has now been adopted by the Russian Government, and by our navy in its specifications for smokeless powder; but they have, I think unwisely, selected a cellulose nitrate containing 12.5 per cent or less of nitrogen instead of that of the highest nitration.
This work was completed, a factory established, and the processes well marked out when I left the torpedo station in 1892. Besides this, there were then already commercial works established elsewhere in this country for the manufacture of the nitroglycerin nitrocellulose powders of the ballistite class, while large quantities of many varieties could be easily procured abroad. Considering these facts, and that France and Germany had already adopted smokeless powders in 1887, that Italy adopted one in 1888, and England about the same time, it is unpardonable that our services should not yet have adopted any of the smokeless powders available when we were drawn into the conflict with Spain. Besides their use as ballistic agents, gun cotton, dynamite, and explosive gelatin in their ordinary condition have found employment and been adopted as service explosives in military and naval mining, as their great energy and the violence with which they

Launching Patrick Torpedo from the Ways.
explode, even when unconfined, especially adapt them for use in the various kinds of torpedoes and mines which are in vogue in the service.
One form of these torpedoes was attached to the end of a spar or pole which was rigged out from the bow of a launch or vessel so that it could be thrust under the enemy's vessel, and the detonators of such spar torpedoes were not only connected with electric generators, so that they could be fired at will, but they, in common with mines, were frequently provided with a system of levers so arranged that the enemy's vessel fired the torpedoes and mines automatically as it came in contact with the levers. It was with such a contact-spar torpedo, containing thirty-three

Patrick Torpedo under way. Moving at the rate of twenty-three knots per hour.
pounds of gun cotton, that the schooner Joseph Henry was blown up in Newport Harbor in 1884.
There are many types of the automobile torpedo. Among them the Hall, Patrick,-Whitehead, and Howell may be cited. The first three are propelled by the energy resident in compressed gases; the Howell by the energy stored in a heavy fly wheel, which also, by acting on the gyroscopic principle, serves to maintain the direction imparted to the torpedo as it is launched. The Hall, Whitehead, and Howell are launched from tubes or guns by means of light powder charges, and are independent of exterior control after launching. The Patrick is launched from ways, and is controlled from the shore or boat by a wire through which an electric current may be sent to its steering mechanism. The charges are quite variable, but the war heads of the larger torpedoes contain as much as five hundred pounds of gun cotton.
[To be concluded.]
